PEGylated cystathionine beta synthase for enzyme therapy for treatment of homocystinuria
A technology for synthesizing enzymes and cystathionine, which is applied in the direction of enzymes, lyases, and drug combinations, and can solve problems such as limitations, anxiety about long-term medical consequences of diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0375] Example 1. Experimental protocol
[0376] A. Fermentation
[0377] Expression vectors with sequences encoding truncated human CBS were transformed into B1-21(DE3) E. coli bacteria, and bacteria from kanamycin resistant clones were grown in cells with 30 μg / mL kanamycin. Grow in 5 ml Luria-Bertani (LB) medium overnight at 37°C on a rotary shaker at 275 rpm. 1 mL of the overnight culture was added to 100 mL of TB broth (Terrific Broth) medium with 30 μg / mL kanamycin and grown overnight. 10 ml of the preculture was then added to 1 liter of TB medium containing 0.001% thiamine-HCl pH 8.0, 0.0025% pyridoxine-HCl pH 8.0, 0.3 mM delta-ALA pH 8.0, 150 μM chloride iron, 30 μg / mL kanamycin. Cultures were then grown at 30°C on a rotary shaker at 275 rpm until OD600 reached a value of about 0.6-0.7, and protein expression was induced by addition of 1 mM IPTG. Continue to ferment for an additional 16 hours. Cells were harvested by centrifugation at 6000 relative centrifugal f...
Embodiment 2
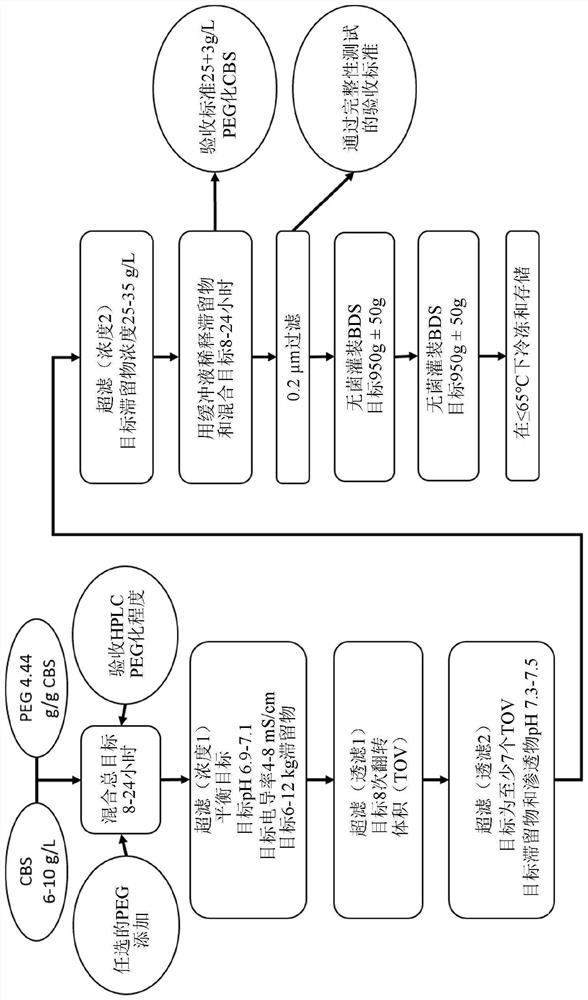
[0423] Example 2. Measurement of Reduction in PEGylation Levels in Pharmaceutical Formulations
[0424] Production of drug product formulations with varying degrees of dePEGylation for accelerated stability studies by incubating drug products at 25°C for two days, one month or six months under "Good Manufacturing Practice" (GMP) conditions for drug products to proceed. Store control samples within the recommended temperature range, i.e. below -65°C.
[0425] Differences in PEGylation between formulations were confirmed by reverse phase high performance liquid chromatography (RP-HPLC). Ten highly PEGylated and less PEGylated species were distinguished, as well as CBS without PEGylation and the heme cofactor (P10) released after enzymatic denaturation. The degree of dePEGylation in each sample was determined by comparing the relative areas of peaks corresponding to variably PEGylated or fully dePEGylated species. The RP-HPLC results are shown in Table 5. "CBS" is the nativ...
Embodiment 3
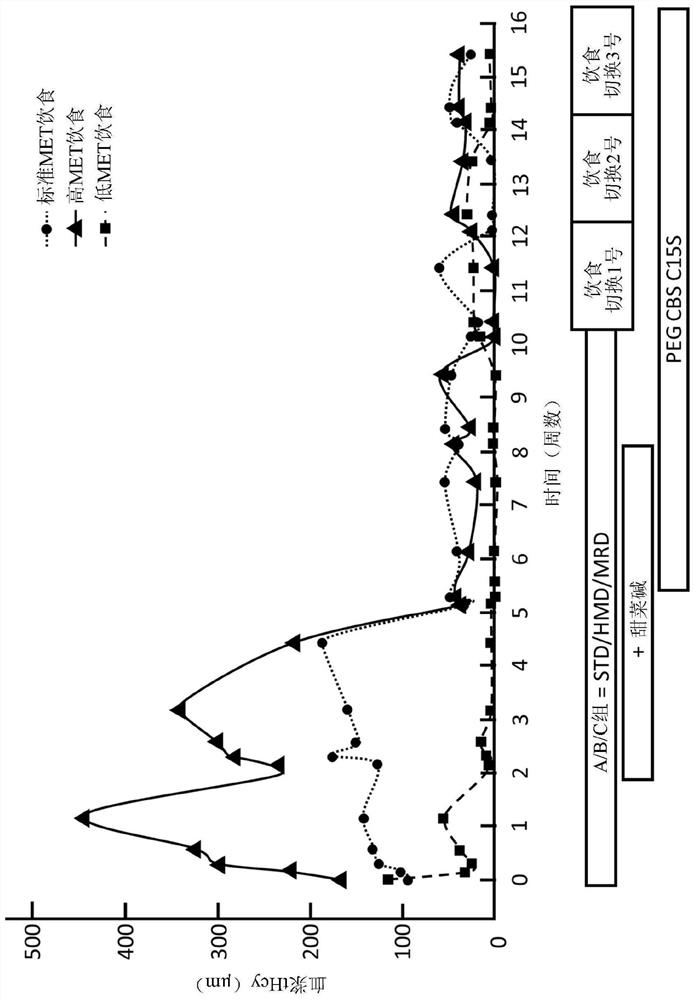
[0430] Example 3. Efficacy of formulations with reduced levels of PEGylation in mice
[0431] To assess bioequivalence of the drug product and its dePEGylated formulation, efficacy was analyzed in I278T CBS- / -(I278T- / -) mice, a model that reproduces the biochemical sequelae of CBSDH, namely Abnormal plasma levels of methionine (Met) metabolites, including elevation of homocysteine (Hcy) and inhibition of cysteine (Cys). Plasma aminothiols (total Cys and Hcy) and residual amino acids as well as SAM and SAH were measured by LC-MS / MS as described in: Arning et al. (2016) Methods Mol Biol 1378, 255-262, which are incorporated herein by reference in their entirety.
[0432] I278T CBS- / - (I278T- / -) mice lack the mouse CBS gene and express the I278T mutant human CBS gene carrying the most widely found pathogenic mutation in CBSDH patients. These mice express about 2%-3% of wild-type CBS activity and have a homocystinuric (HCU) phenotype (see Wang, et al. (2005) Human Molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com