Application of Rassf6 as marker of Alzheimer's disease
A technology for Alzheimer's disease markers, applied in the application field of Rassf6 as a marker for Alzheimer's disease, can solve problems such as unsatisfactory effects of treatment plans, achieve novel research methods, good repeatability, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
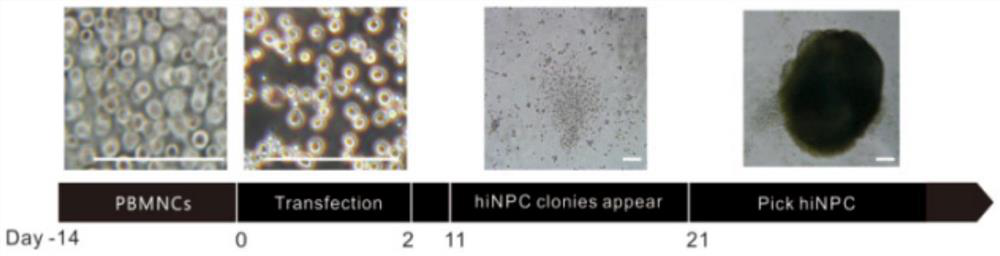
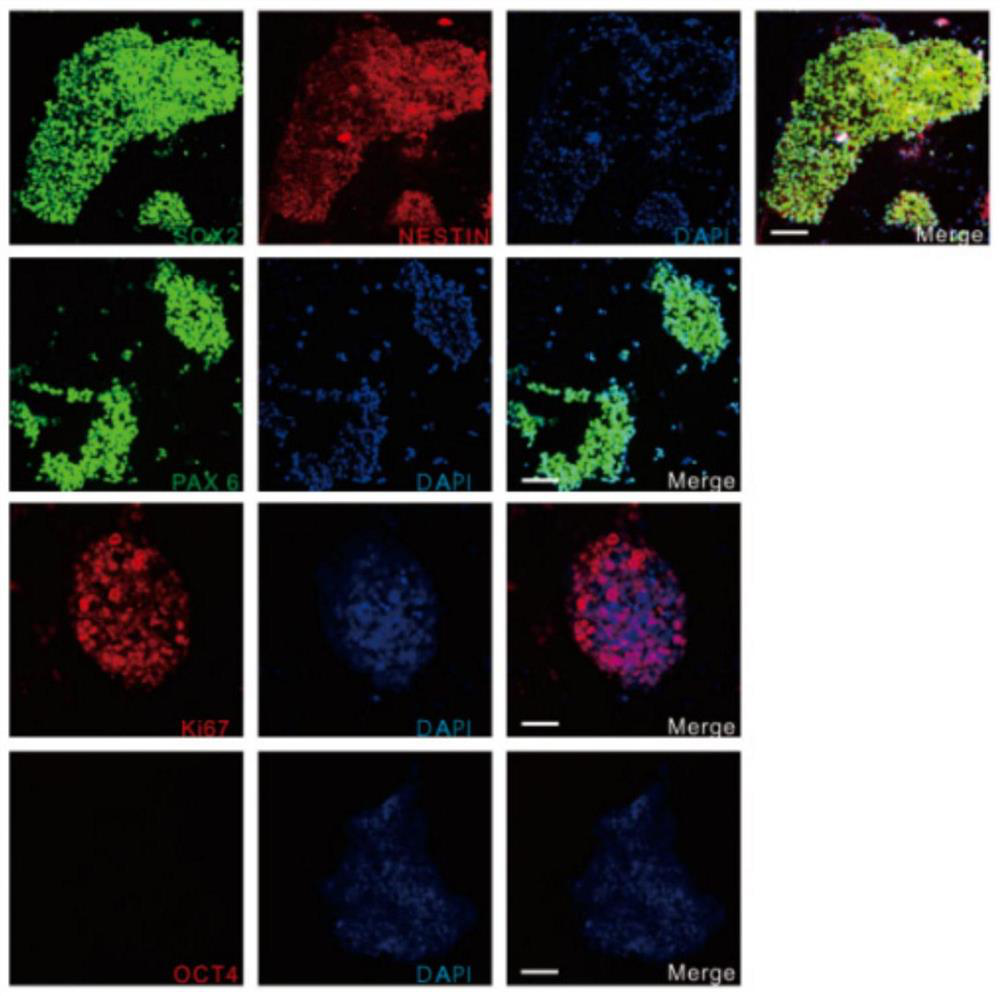
[0072] The preparation of GABAergic neural precursor cells includes the following steps, wherein the reprogramming process of steps 1-6 is as follows figure 1 As shown, the differentiation process of step 7 is as follows figure 2 shown:
[0073] (1) Screen healthy young adults, sign the consent form for sample collection from the medical biobank, and collect blood from the right forearm and cubital vein of the volunteer under sterile conditions, about 10 mL, with EDTA-K2 anticoagulation.
[0074] (2) Peripheral blood and PBS were mixed at a ratio of 1:2, human lymphocyte separation medium was added, centrifuged at 750 g for 30 min, the middle cloud layer was taken, and mononuclear cells (PBMNC) were extracted.
[0075] (3) Cells are 0.5-1×10 6 Density was seeded in 6-well plates, cultured in mononuclear cell expansion medium, and the medium was changed every two days to expand to obtain PBMNCs.
[0076] (4) When the mononuclear cells were expanded to the 14th day, OCT3 / 4, ...
Embodiment 2
[0084] Model assessment of the therapeutic effect of MGE on AD.
[0085] 1. Method.
[0086] The γ-aminobutyric acid-producing neural precursor cells (MGE) were prepared according to the method of Example 1 above, and 5 × 10 6 AD double transgenic model mouse APP / PS1 mice (purchased from Changzhou Cavens Experimental Animal Center) were injected into the right hippocampus of the brain localizer, and the wild-type WT mice were used as the blank. Control group. details as follows:
[0087] 1.1 Preparation of labeled GABAergic neural precursor cells
[0088] Utilize the lentiviral vector pLV-CMV-MCS-EGFP-3FLAG-IRES-puro (source: Guangzhou Jidan Biotechnology Co., Ltd.) to transfect the cultured hiNPC, make it with green fluorescent label, and refer to the above Example 1 Methods, GFP-labeled hiNPCs were differentiated toward MGE to obtain γ-aminobutyric acid-ergic neural precursor cells with green fluorescent labels.
[0089] 1.2 Surgery
[0090] APP / PS1 double-transgenic m...
Embodiment 3
[0122] Validation of Rassf6 factor as a disease target in AD pathogenesis.
[0123] 1. Method.
[0124] 1.1 Serum content of clinical samples
[0125] With the consent of the Ethics Committee of the People's Hospital of Guangxi Zhuang Autonomous Region, blood samples from AD patients and normal people were collected, among which AD samples were divided into CLU site mutation group and non-CLU site mutation group. Serum was collected to determine Rassf6 content by Elisa method.
[0126] 1.2 Cell culture supernatant content
[0127] PBMNCs from AD patients and normal people with CLU site mutation were taken and reprogrammed into hiNPCs according to the method of Example 1. The content of Rassf6 in the supernatant of AD-hiNPC cells and Cont-hiNPC cells (normal human origin) was compared.
[0128] 2. Results.
[0129] The content of Rassf6 factor in clinical serum samples is as follows: Figure 40 As shown, among them, Cont, normal group; Non-CLUmutation AD, AD patient group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com