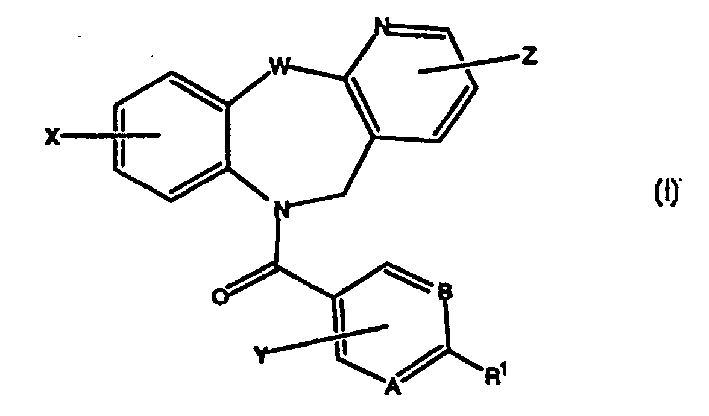
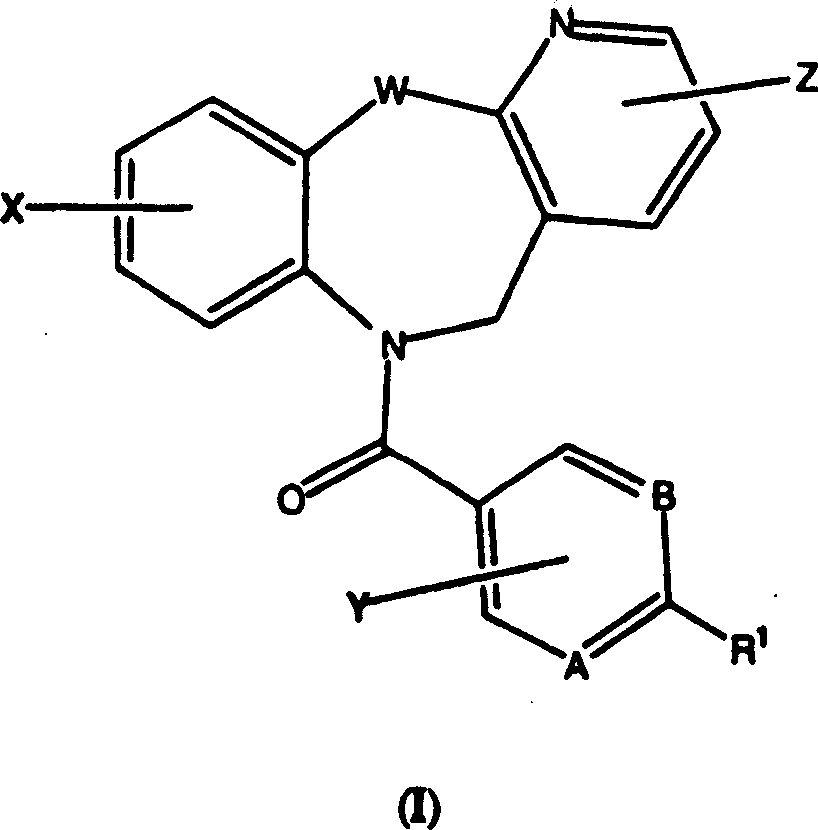
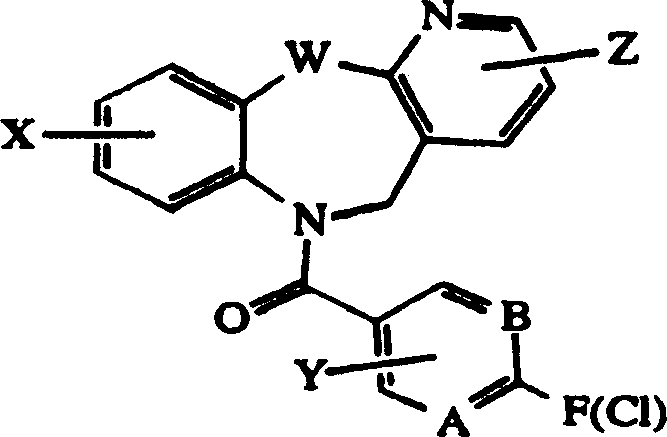
Tricyclic vasopressin agonists
A kind of technology of heterocyclic group and trifluoromethyl group is applied in the field of pyridotricyclic compounds or their medicinal salts to achieve the effect of good oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0257] [2-Chloro-4-(3-methyl-pyrazol-1-yl)-phenyl]-(5,11-dihydro -pyrido[2,3-b][1,5]benzodiazepine-6-yl)-methanone
[0258] Step A. 6, 11-dihydro-5H-pyrido[2,3-b][1,5]benzodiazepine-5-one and hydrochloric acid 1:1 salt
[0259] A mixture of 1,2-phenylenediamine (52 g, 480 mmol) and chloronicotinic acid (76 g, 482 mmol) in cyclohexanol (480 mL) was refluxed under nitrogen for 2.5 hours. Precipitation appeared shortly after heating was started. With vigorous stirring, the hot reaction mixture was carefully poured into ice-cooled dichloromethane (1000 mL). The semisolid material was collected, washed well with dichloromethane and dried in vacuo to yield 98.9 g (83%) of the title compound, which was used in the next reaction without purification.
[0260]Step B. 6,11-Dihydro-5H-pyrido[2,3-b][1,5]benzodiazepine
[0261] The diborane dimethyl sulfide complex (35 mL) was added via syringe to the 6,11-dihydro-5H-pyrido[2,3-b][1,5 ] Benzodiazepine-5-one and hydrochloric acid 1:...
Embodiment 2
[0273] [2-Chloro-4-(5-methyl-pyrazol-1-yl)-phenyl]-(5,11-di Hydrogen-pyrido[2,3-b][1,5]benzodiazepin-6-yl)-methanone
[0274] The fraction (0.543 g) containing the mixture of 3-methyl and 5-methylpyrazole regioisomers obtained in step E of Example 1 was passed through a flash chromatography column (silica gel Merck-60, eluent: toluene-acetic acid ethyl ester 90:10, followed by toluene-ethyl acetate-acetonitrile 90:10:5), to obtain 0.327 g of the 3-methyl isomer in Example 1 already described and 0.105 g of the title compound in diethyl ether- Sonicated in hexane, amorphous solid.
[0275] NMR (DMSO-d 6 , 400MHz): δ2.27(s, 3H), 4.16 and 5.45(dd, 2H, CONCH 2 ), 6.25(m, 1H), 6.54(m, 1H, pyrazole CH), 6.79(m, 2H), 7.01(m, 1H), 7.26(m, 1H), 7.40-7.54(mm, 3H), 7.61 (m, 1H), 8.11 (m, 1H, pyrazole CH), 9.56 (s, 1H, NH)
[0276] MS [EI, m / z]: 415 / 417 [M] + , 219 / 221, 196
Embodiment 3
[0278] [2-Bromo-4-(3-methyl-pyrazol-1-yl)-phenyl]-(5,11-di Hydrogen-pyrido[2,3-b][1,5]benzodiazepin-6-yl)-methanone
[0279] Step A. 2-Bromo-4-fluorobenzoyl chloride
[0280] 2-Bromo-4-fluorobenzoic acid (6.87 g, 31.37 mmol) in dichloromethane containing a few drops of dimethylformamide was treated dropwise with a 2M solution of oxalyl chloride in dichloromethane (1.16 eq) under nitrogen. Methane (70 mL) suspension. After gas evolution had ceased, the reaction mixture was refluxed for an additional 25 minutes, then the solution was evaporated to dryness under vacuum. The crude acid chloride was used directly in the next step.
[0281] Step B. (2-Bromo-4-fluorophenyl)-(5,11-dihydro-pyrido[2,3-b][1,5]benzodiazepin-6-yl)-methanol ketone
[0282] Add 6,11-dihydro-5H-pyrido[2,3-b][1,5]benzodiazepine (5.15 g, 26.1 mmol) from Example 1, Step B under nitrogen to Potassium carbonate (7.95 g, 57.51 mmol) was added to a solution of methylformamide (70 mL). The mixture was coole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap