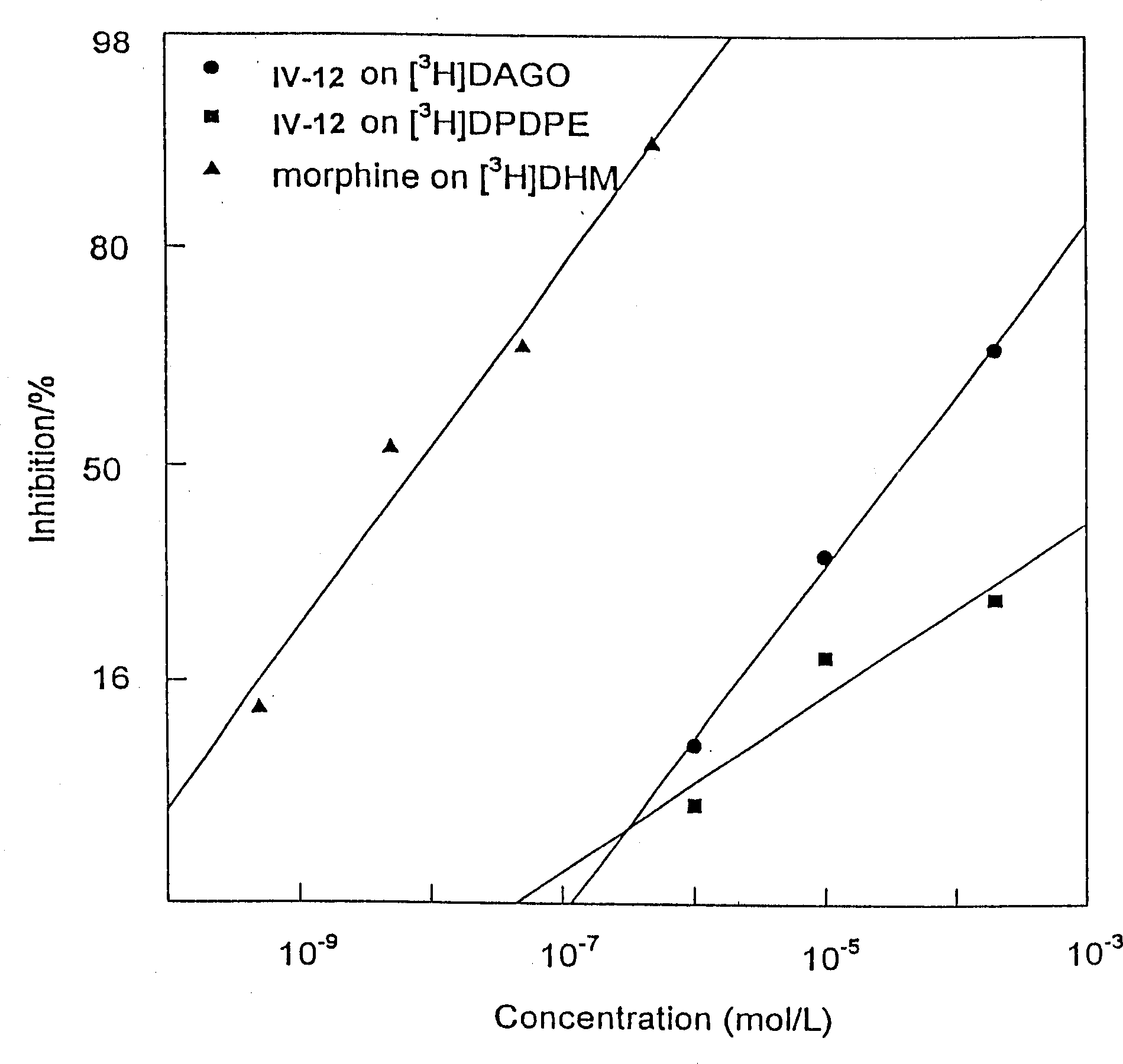
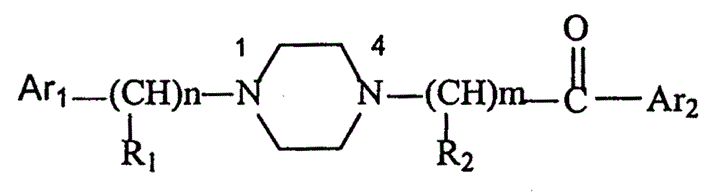Aralkylone pipeazine derivative and its application
一种芳烷酮哌嗪、衍生物的技术,应用在芳烷酮哌嗪衍生物领域,能够解决胃蠕动减少等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1IV-1
[0098] Example 1IV-1 N 1 -Benzyl-N 4 -Phenylmethylpiperazine hydrochloride
[0099] N-benzylpiperazine hydrochloride 3.98g (16mmol), acetophenone chloride 2.97g (19.2mmol) and anhydrous K 2 CO 3 7.73g (56mmol) was synthesized in acetone according to general method two to obtain 4.5g of white solid, yield 75%, mp 238-9°C.
[0100] Elemental analysis: C 19 H 22 N 2 O·2HCl·H 2 O (theoretical value%: C 59.31, H 6.8, N 7.27, Cl 18.4; experimental value% C 59.38, H 6.51, N 7.12, Cl 18.23)
[0101] IR(KCl): ν2950, 1710, 1600, 1500cm -1 .
[0102] 1 HNMR(DMSO-d 6 ): δ3.58 (m, 8H, piperazine-H), 4.50 (s, 2H, PhCH 2 ), 5.22(s, 2H, COCH 2 N), 7.40-8.10 (m, 10H, ArH).
[0103] MS: m / z 294(M + )
Embodiment 2IV-2
[0104] Example 2IV-2 N 1 -P-chlorobenzyl-N 4 -(1-Benzoylethyl)piperazine hydrochloride
[0105] Take N-p-chlorobenzylpiperazine hydrochloride 5g (20mmol), anhydrous KHCO 3 (70mmol) and 3.96ml (26mmol) of 2-bromopropiophenone, reacted at reflux in 40ml of absolute ethanol for 8 hours. After treatment according to the general method, the product is 6.1g, yield 59.51%, mp260-262℃
[0106] Elemental analysis: C 20 H 23 ClN 2 O·2HCl·H 2 O (theoretical value%: C 55.37, H 6.27, N 6.46; experimental value% C55.01, H 6.01, N 6.41)
Embodiment 3IV-3
[0107] MS: m / z 342(M + ) Example 3IV-3 N 1 -P-chlorobenzyl-N 4 -Phenylmethylpiperazine hydrochloride
[0108] Take 5g (20mmol) of N-p-chlorobenzylpiperazine hydrochloride, 5.58ml (40mmol) of triethylamine and 4.08g (26mmol) of chloroacetophenone, reflux for 8 hours in 40ml of absolute ethanol, press the pass After the second treatment method, the product is 5.2g, the yield is 59.51%, mp256-258℃
[0109] Elemental analysis: C 19 H 21 ClN 2 O·2HCl·H 2 O (theoretical value%: C 55.37, H 6.27, N 6.46; experimental value% C55.01, H 6.01, N 6.41)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com