Leptospira hemolysin and its code sequence
A leptospira and hemolysin technology, which is applied in the fields of biotechnology and medicine, and can solve problems such as leptospira hemolysin that have not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Bacterial culture
[0074] 1) Culture medium: use EMJH liquid medium to prepare 1000 ml of 5× EMJH medium, and weigh 50 g of BSA. Anhydrous sodium acetate 0.5g, sodium pyruvate 1g, ferrous sulfate 0.25g, 1.5% magnesium chloride 3.5ml, 0.4% zinc sulfate 5ml, 0.15 copper sulfate 1ml, disodium hydrogen phosphate 12.6g, potassium dihydrogen phosphate 1.5 g, sodium chloride 5g, ammonium chloride 1.25g, 0.05% VB 12 2ml, VB 1 0.025g, adjust the pH to 7.2-7.6, set the volume to 1000ml, and sterilize by filtration through nitrocellulose membranes with 0.7, 0.45, and 0.2nm pore sizes, respectively. When used, dilute it 5 times with sterile water.
[0075] 2) Culture conditions: 30° C., 100 rpm shaking culture for 40 hours.
[0076] 3) Strains: Leptospira interrogans jaundice and haemorrhagic group Lai type Lai strain.
Embodiment 2
[0078] Nucleic acid and amino acid sequences of hemolysin
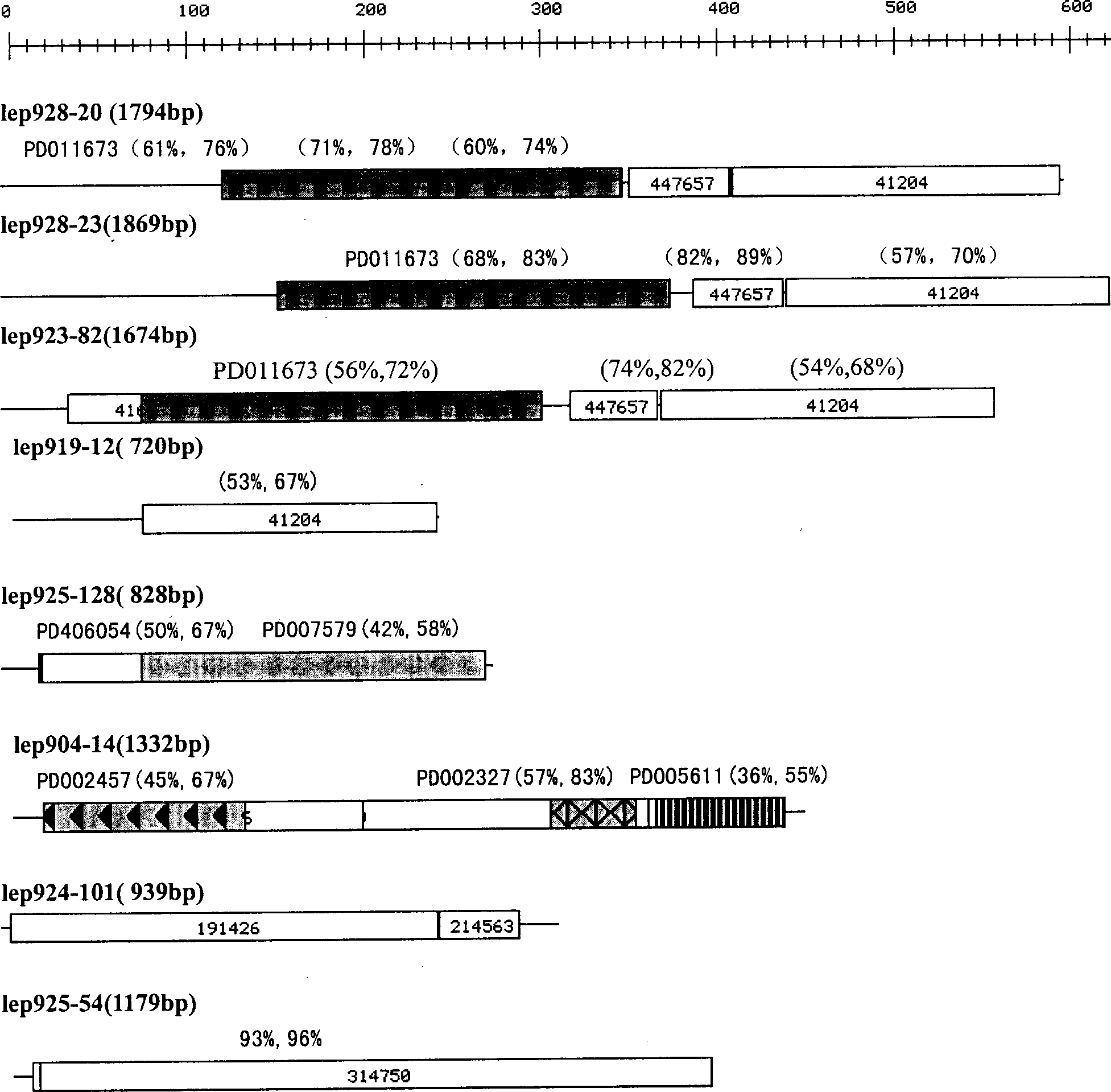
[0079] Leptospira DNA was extracted by conventional methods to construct a library. Determining the complete sequence of the genome. Based on computer analysis, primers were synthesized, and the extracted Leptospira DNA was used as a template for PCR amplification to obtain the ORF sequence of hemolysin. Sequencing verification again, consistent with the genome sequence. These ORFs encode polypeptides having the amino acid sequences shown in SEQ ID NO: 2, 4, 6, 8, 10, 12, 14, 16. The basic information of the eight hemolysins of the present invention is shown in the table below.
[0080] name
[0081] Leptospira hemolysin No.1-8 of the present invention has certain homology with the hemolysin of other bacteria, and has the specific structure domain of hemolysin ( figure 1 ).
Embodiment 3
[0083] Protein
[0084] The Leptospira hemolysin gene was cloned into the fusion expression vector pET28b (Novagene Company) by conventional genetic engineering method, and Escherichia coli BL21 (DE3) was selected as the recipient bacteria for fusion expression. After fermentation under conventional conditions, the recombinant bacterial protein was extracted, and then subjected to SDS-PAGE electrophoresis, and the molecular weight was basically the same as the predicted value. The hemolysin (fusion protein with His-tag) expressed in Escherichia coli was separated and purified by Ni-NTA column to obtain pure Leptospira hemolysin (fusion protein).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap