Anti-AIDS compound, its preparing method and pharmaceutical composition with anti-AIDS compound as active component and use thereof
A kind of technology of compound and medicine, applied in the field of application in the preparation of anti-AIDS medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
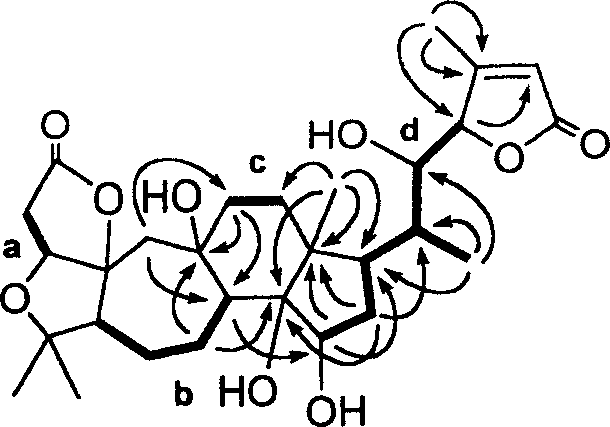
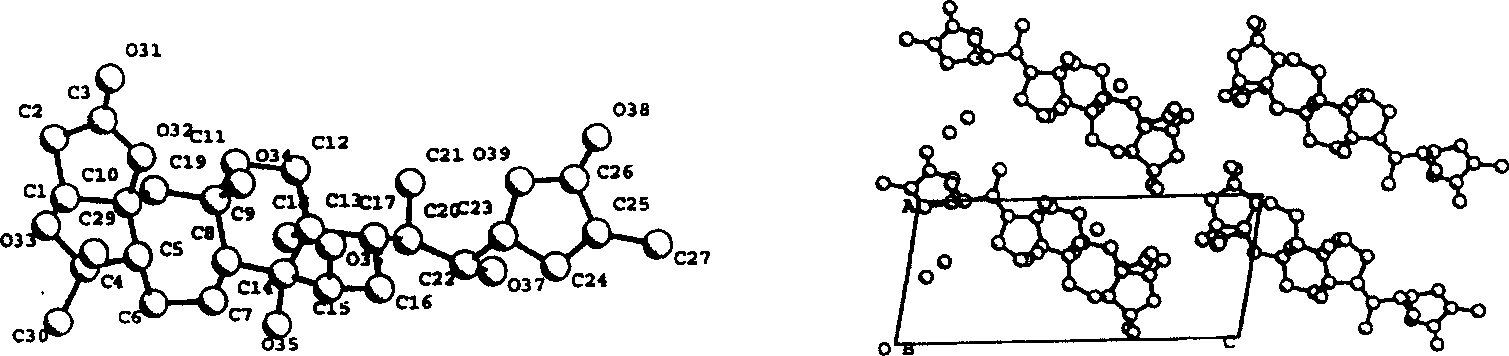
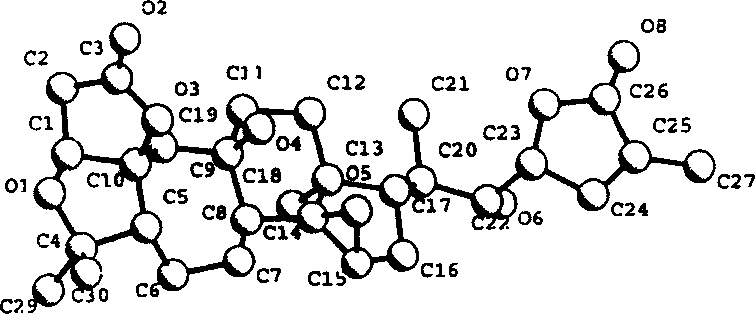
[0034] Schizandrin B(1) (1,4-epoxy-9β,14α,15β,22α-tetrahydroxy-3,4:9,10-seco-28-nor-cycloartane-24-en-1,3:23 , 26-diolide, 1) (hereinafter referred to as the preparation of compound 1):
[0035] Dried Schisandra micrantha A.C.Smith stems and leaves 6.8kg, crushed, soaked in acetone aqueous solution at room temperature for 3 times (about 25 liters each time), combined the extracts, distilled off the acetone under reduced pressure, let stand overnight, and filtered out the sediment The pigment, the filtrate was first extracted 2 times with petroleum ether, and then extracted 3 times with ethyl acetate. After recovering the solvent, the ethyl acetate part (170g) was obtained. The ethyl acetate part was mixed with 300g of crude silica gel (80-100 mesh), and 1.4kg of silica gel (100-200 mesh) was roughly separated on a thick and short silica gel column, and washed with a gradient of chloroform:acetone (1:0→6:4) Each 1000ml is a fraction, a total of 300 parts. TLC Monitoring Ident...
Embodiment 2
[0082] Schisandrin C(2)
[0083] (1, 4:14, 15-diepoxy-9β, 22α-dihydroxy-3, 4:9, 10-seco-28-nor-cyclartane-24-en-1, 3:23, 26-diolide, 2)( Hereinafter referred to as compound 2) preparation:
[0084] Dried Schisandra micrantha A.C.Smith stems and leaves 6.8kg, crushed, soaked in acetone aqueous solution at room temperature for 3 times (about 25 liters each time), combined the extracts, distilled off the acetone under reduced pressure, let stand overnight, and filtered out the sediment The pigment, the filtrate was extracted 3 times with ethyl acetate, after recovering the solvent, the ethyl acetate part (170g) was obtained. The ethyl acetate part was mixed with 300 g of crude silica gel (80-100 mesh), and 1.4 kg of silica gel (100-200 mesh) was roughly separated on a thick and short silica gel column, and washed with a gradient of chloroform: acetone (1:0 → 6:4). Each 1000ml is a fraction, a total of 300 parts. TLC Monitoring Identical fractions were pooled to give 8 major fr...
Embodiment 3
[0093] Using Example 1 or 2 as an active natural lead compound, carry out structural modification to obtain other derivatives represented by formula (1) as active ingredients of the medicine of the present invention.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap