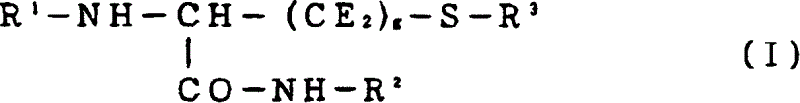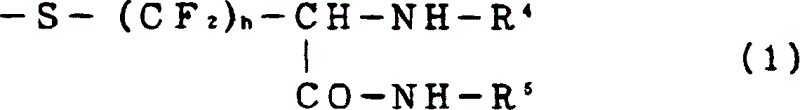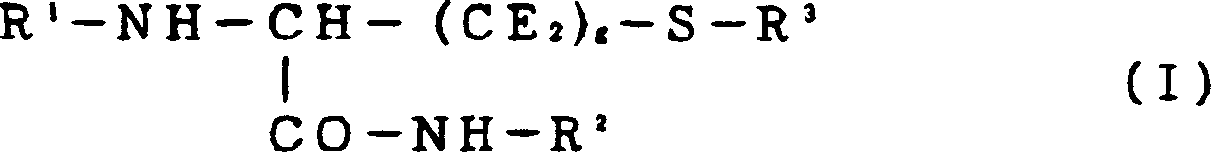Cysteine derivatives
A technology of cysteine and derivatives, which is applied in the field of cosmetics and external skin pharmaceuticals, and can solve problems such as side effects, unsatisfactory effects, and low photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0065] Synthesis Example 1: Synthesis of Cystine Diamide Derivatives
[0066] To 7 ml of acetonitrile, 0.15 g of L-cystine diamide dihydrochloride, 0.21 g of n-hexanoic anhydride, and 0.10 g of triethylamine were sequentially added, and the mixture was stirred at room temperature overnight. The crude crystals obtained by concentrating the reaction solution were purified by high-speed liquid chromatography (HPLC fractionation with an apparatus for high-speed liquid chromatography manufactured by Hitachi Co., Ltd. using "Ina-Tosil ODS-3 column" (manufactured by GL Scientific Corporation)) to obtain N,N'-bis(n-hexanoyl)-L-cystine diamide 0.18g. In the same way, various N,N'-diacyl-L-cystine diamides were obtained. These are novel compounds that are not documented in the literature. The mass spectrometry results of these compounds are listed in Table 1 below.
[0067] New compounds
Synthetic example 2
[0068] Synthesis Example 2: Synthesis of cystine dialkyl ester derivatives
[0069] To 25 ml of acetonitrile, 0.50 g of L-cystine dimethyl dihydrochloride, 0.55 g of n-valeric anhydride, and 0.31 g of triethylamine were sequentially added, and the mixture was stirred at room temperature overnight. The crude crystals obtained by concentrating the reaction solution were purified by the same high-speed liquid chromatography as in Synthesis Example 1, to obtain 0.38 g of N,N'-bis(n-valeryl)-L-cystine dimethyl ester. In the same way, various N,N'-diacyl-L-cystine dialkyl esters or N,N'-diacyl-DL-homocysteinyl dialkyl esters were obtained.
[0070] Secondly, reveal the test example.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap