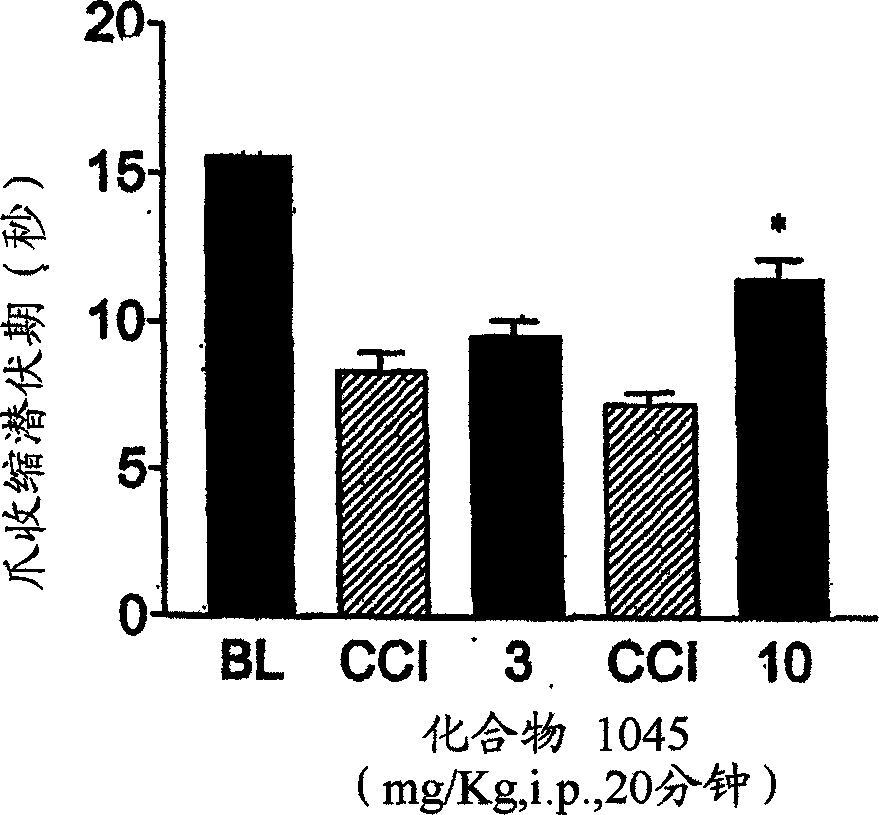
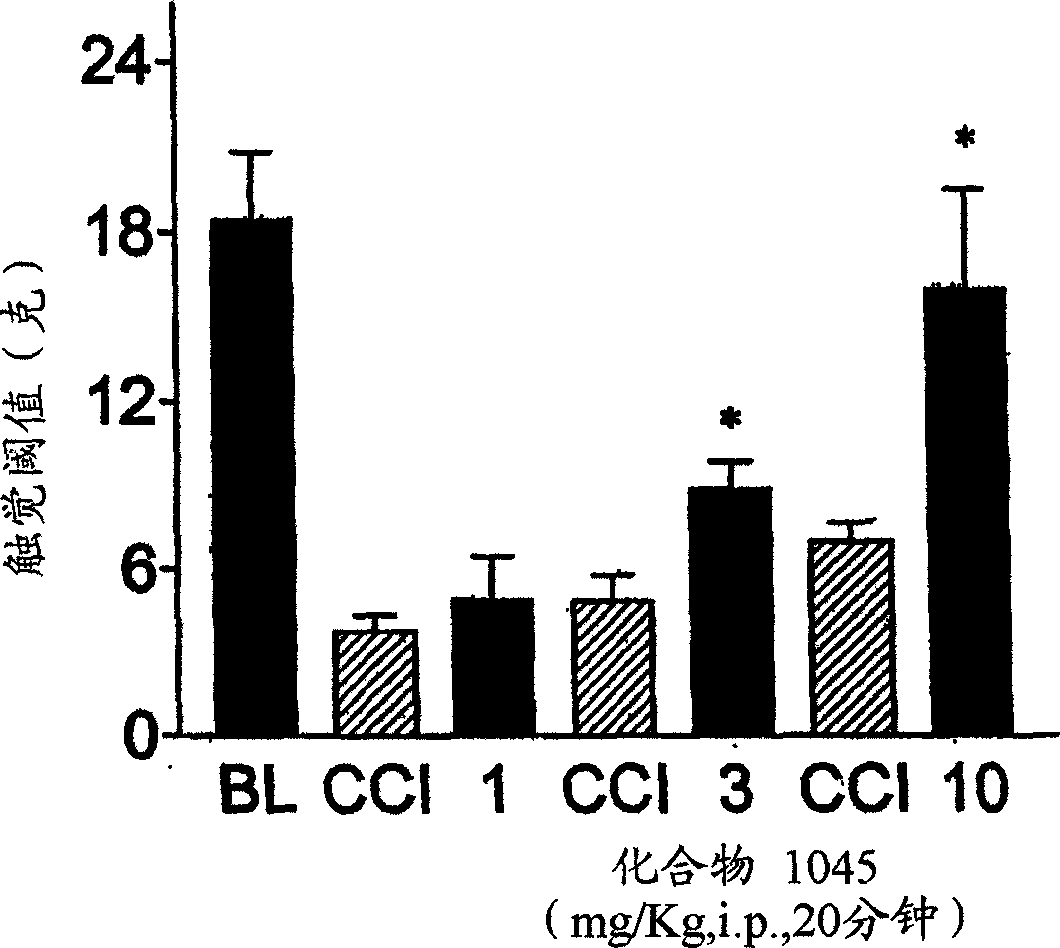
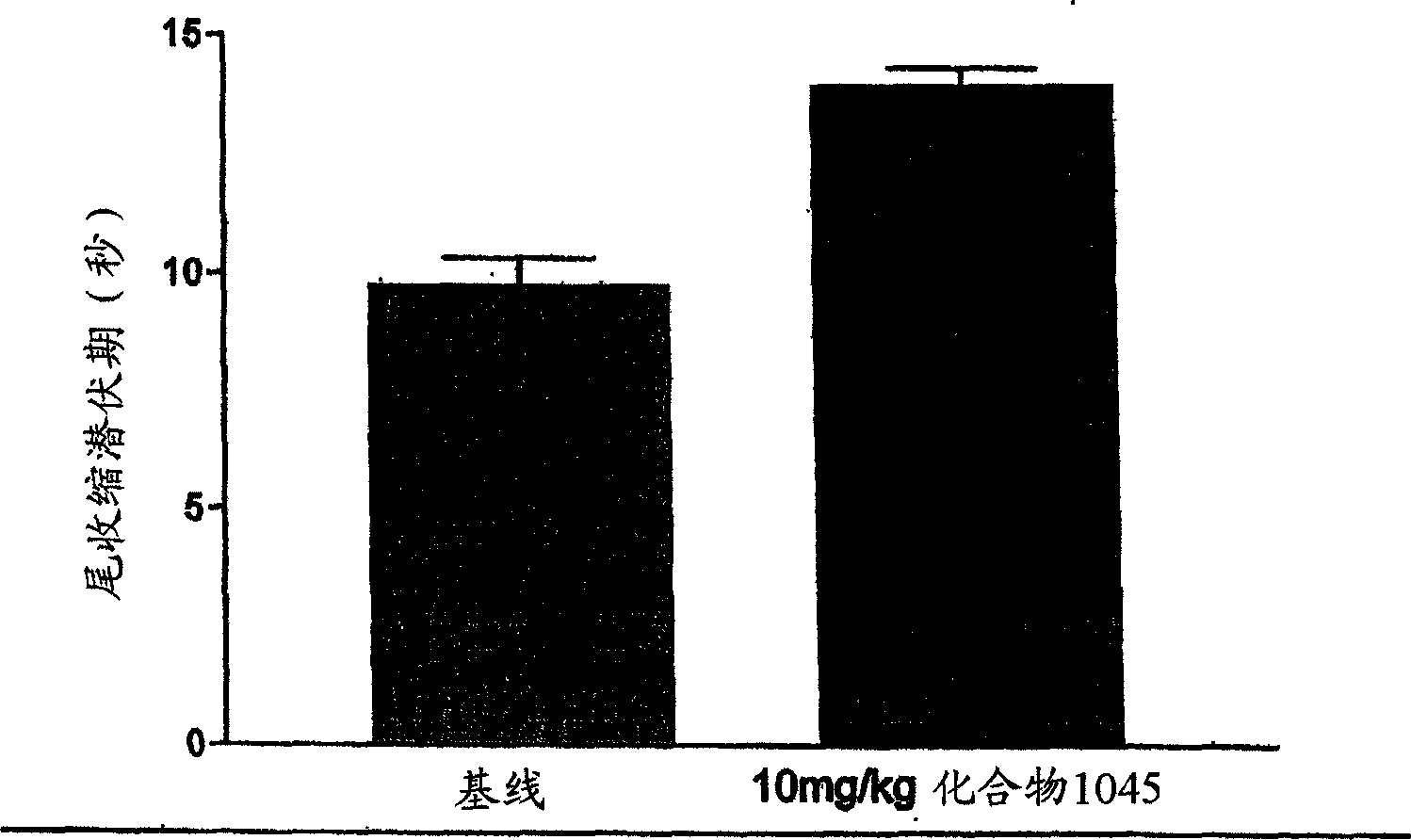
Treating neuropathic pain with neuropeptide FF receptor 2 agonists
A receptor agonist, receptor technology, applied in the field of compounds that selectively interact with this receptor subtype, can solve the problems of onset and inefficiency in all patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0255] Example 1: 1-(4-fluorobenzylideneamino)guanidine hydrochloride (2001)
[0256] Application of 4-fluorobenzaldehyde (5.0 mmol, 621 mg) gave the title compound (2001 ) (534 mg, 49%) as a white powder according to GP1. 1 H NMR (CD 3 OD) δ8.13(s, 1H), 7.85(m, 2H), 7.17(m, 2H); HPLC-MS (ammonium acetate) [M+H]+ = 181.1.
Embodiment 2
[0257] Example 2: 1-[(trifluoromethyl)benzylideneamino]guanidine hydrochloride (2002)
[0258] Application of 3-(trifluoromethyl)benzaldehyde (5.0 mmol, 871 mg) gave the title compound (2002) (643 mg, 48%) as a white powder according to GP1. 1 H NMR (CD 3 OD) δ8.22(s, 1H), 8.17(m, 1H), 8.05(m, 1H), 7.74(m, 1H), 7.65(m, 1H); HPLC-MS (ammonium acetate) [M+H ] + = 231.1.
Embodiment 3
[0259] Example 3: 1-[1-(3-bromophenyl)ethylideneamino]guanidine hydrochloride (2003)
[0260] According to GP1, the title compound (2003) (977 mg, 67%) was obtained as a white powder using 3'-bromoacetophenone (5.0 mmol, 995 mg). 1 H NMR (CD 3 OD) δ8.12 (ap.t, J=1.7Hz, 1H), 7.84 (ddd, J=8.0, 1.7, 1.0Hz, 1H), 7.59 (ddd, J=7.8, 2.0, 1.0Hz, 1H), 7.35(ap.t, J=8.0Hz, 1H), 2.36(s, 3H); HPLC-MS (ammonium acetate) [M+H] + = 255.1, 257.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com