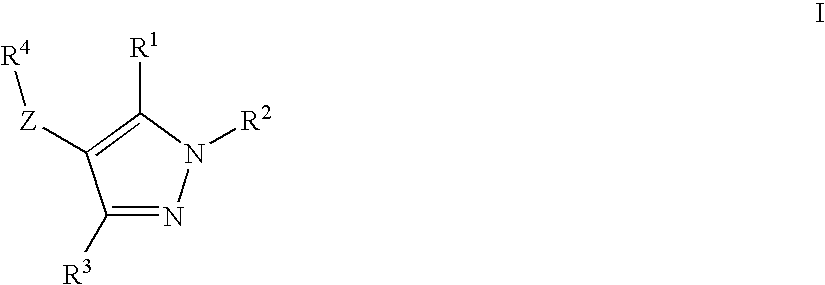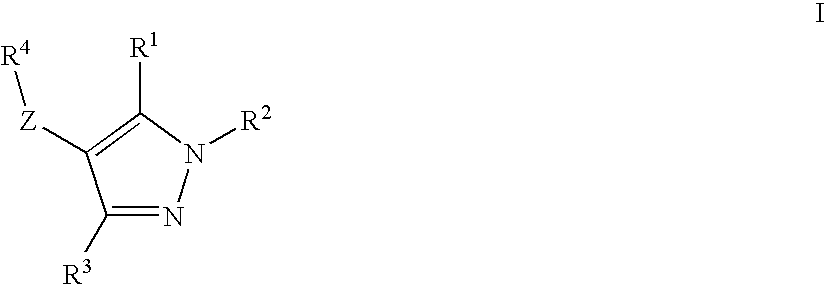Patents
Literature
31 results about "Co morbidity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A co-morbidity is when someone has more than one chronic disease. Co-morbidities are fairly common in people who are older or very sick.
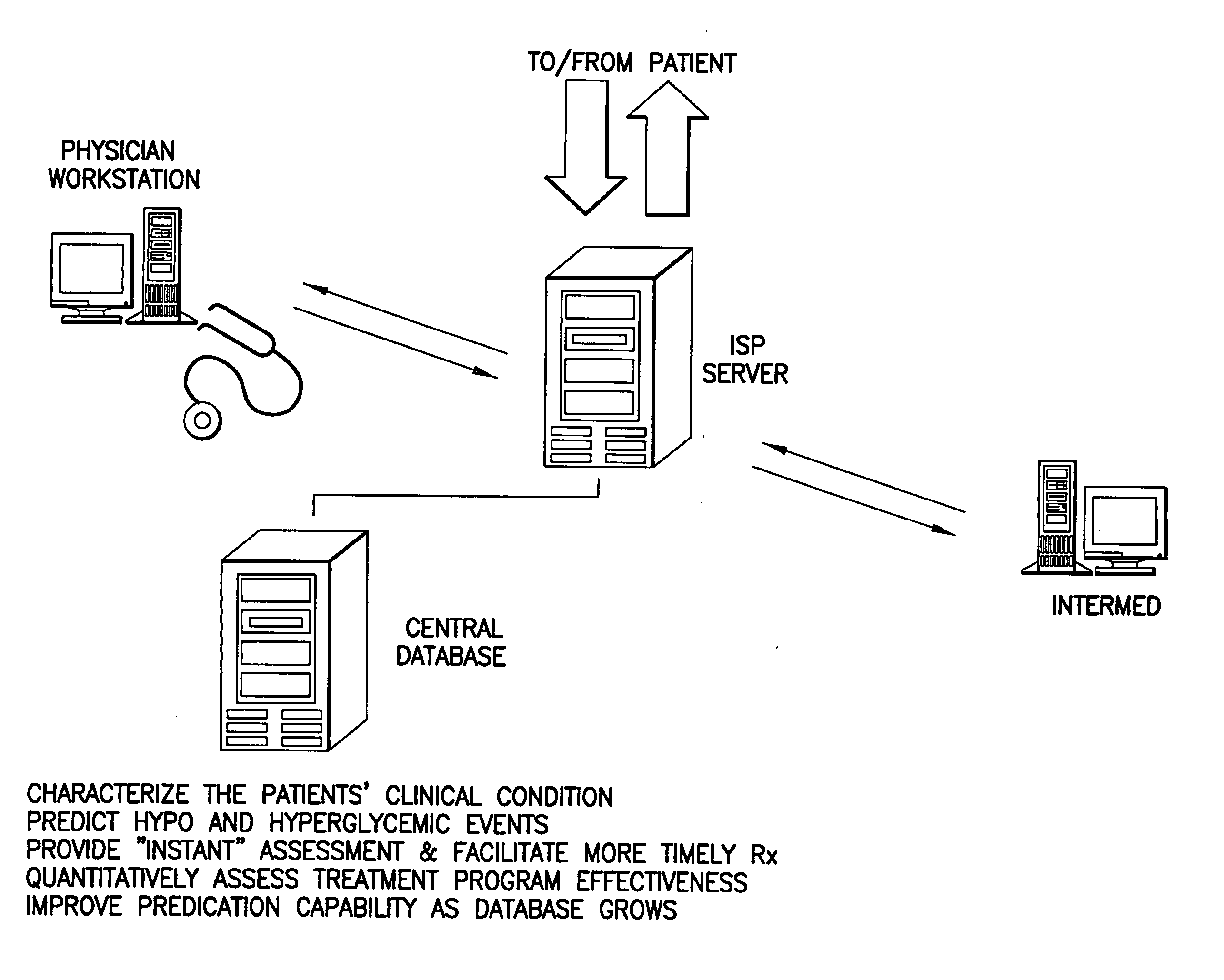
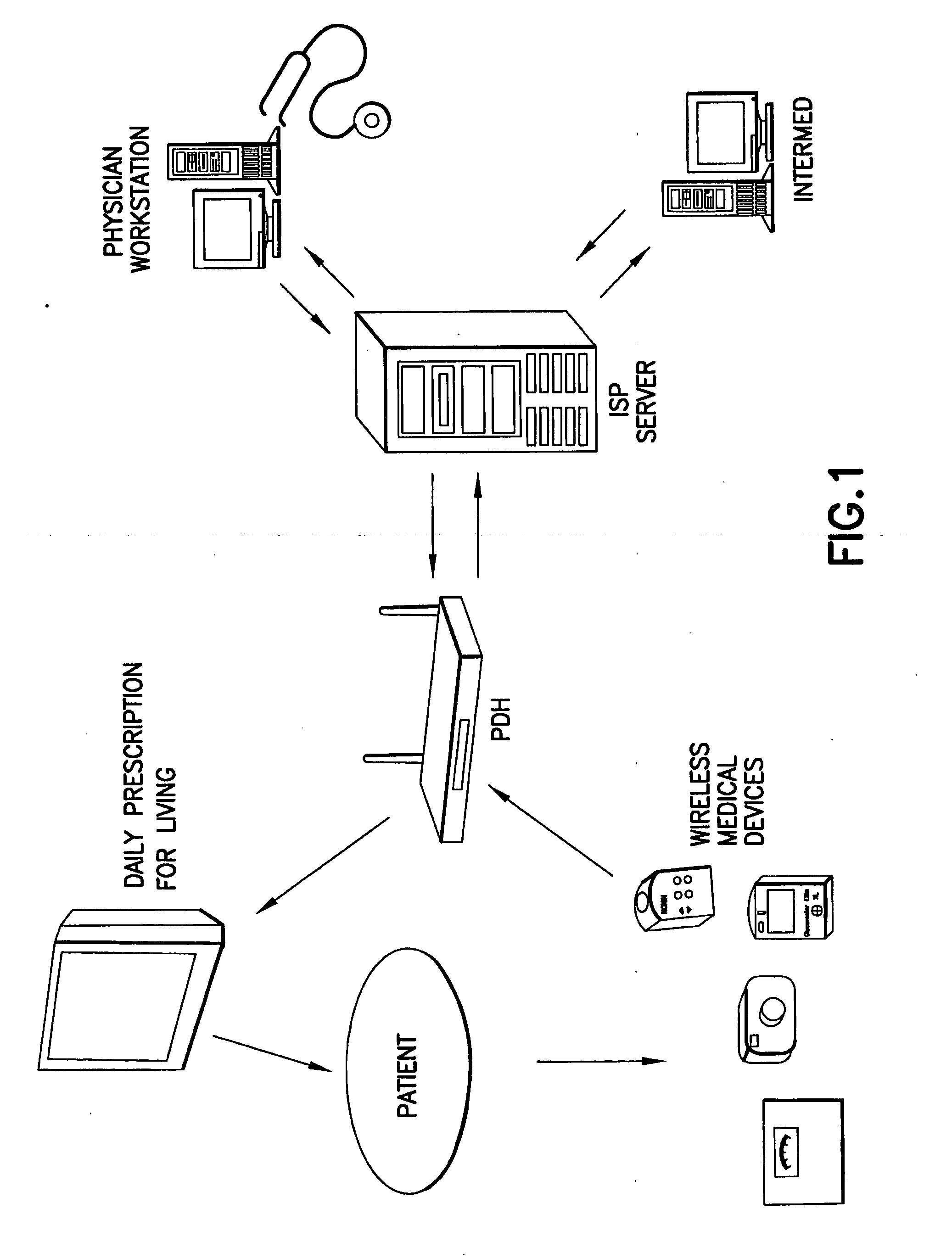
Method and apparatus for real time predictive modeling for chronically ill patients
InactiveUS20060025931A1Low costEasy to manageHealth-index calculationMedical automated diagnosisDiseaseData harvesting
Various embodiments of the present invention are directed to a method and apparatus for improving the care of patients (e.g., chronically ill patients). In one example (which example is intended to be illustrative and not restrictive), the invention may be designed to improve such care through real time output (e.g., periodic, such as hourly, daily, weekly or monthly) to deter development of co-morbidities associated with many chronic diseases. Various embodiments of the invention may improve the care of chronically ill patients by: easing data collection, simplifying data transmission, efficiently interpreting chronically ill patient information, providing time series to form the basis of subsequent analysis, expanding the ability of the chronically ill patient or other user of the invention to understand the relationship between the medical practice and the patient's own lifestyle and / or easing the workload of healthcare providers (such as the patient's physician). In this regard, the invention may help in creating dialogue between chronically ill patients and healthcare providers that otherwise would be impossible.
Owner:INTERMED ADVISOR
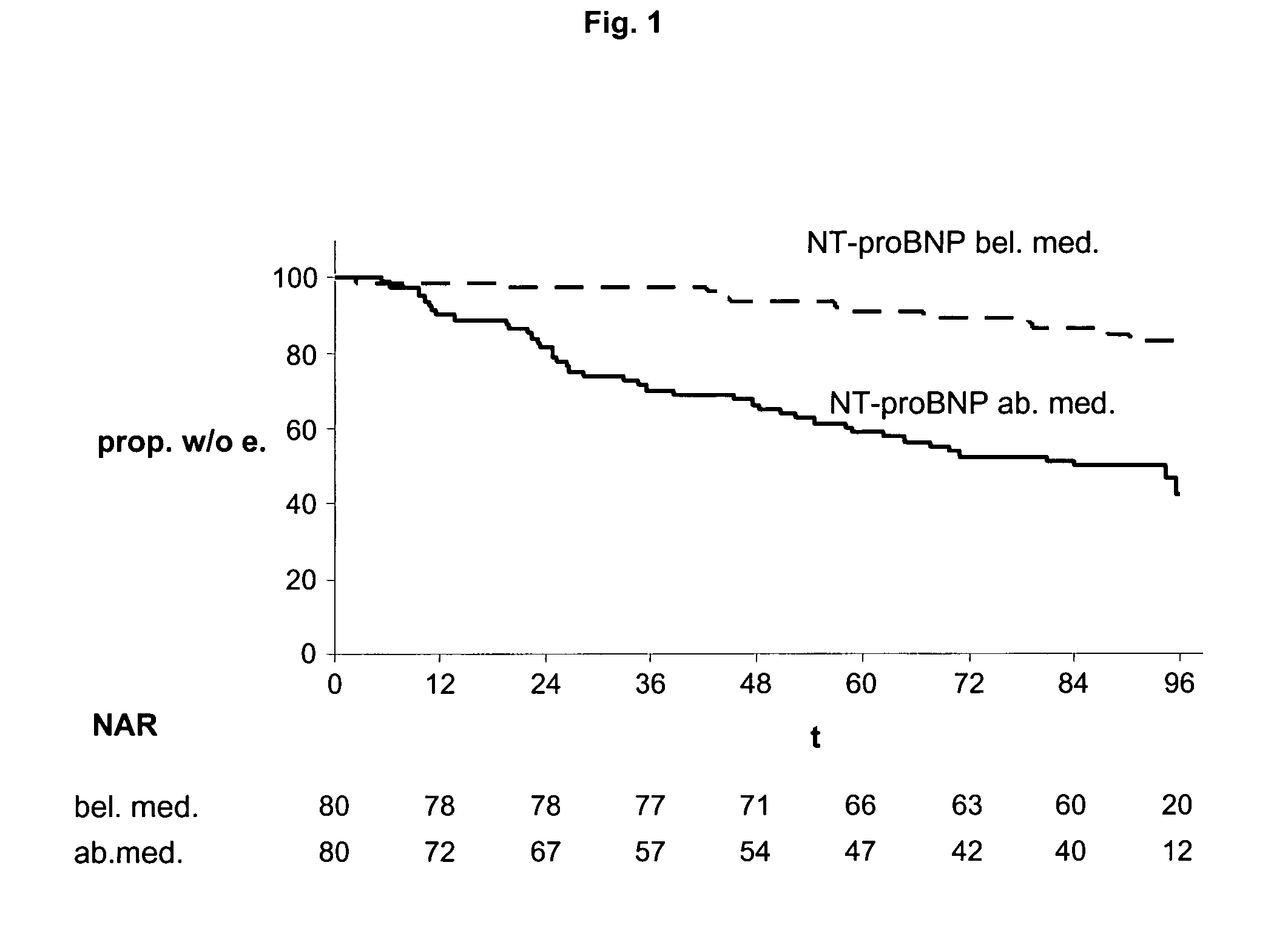
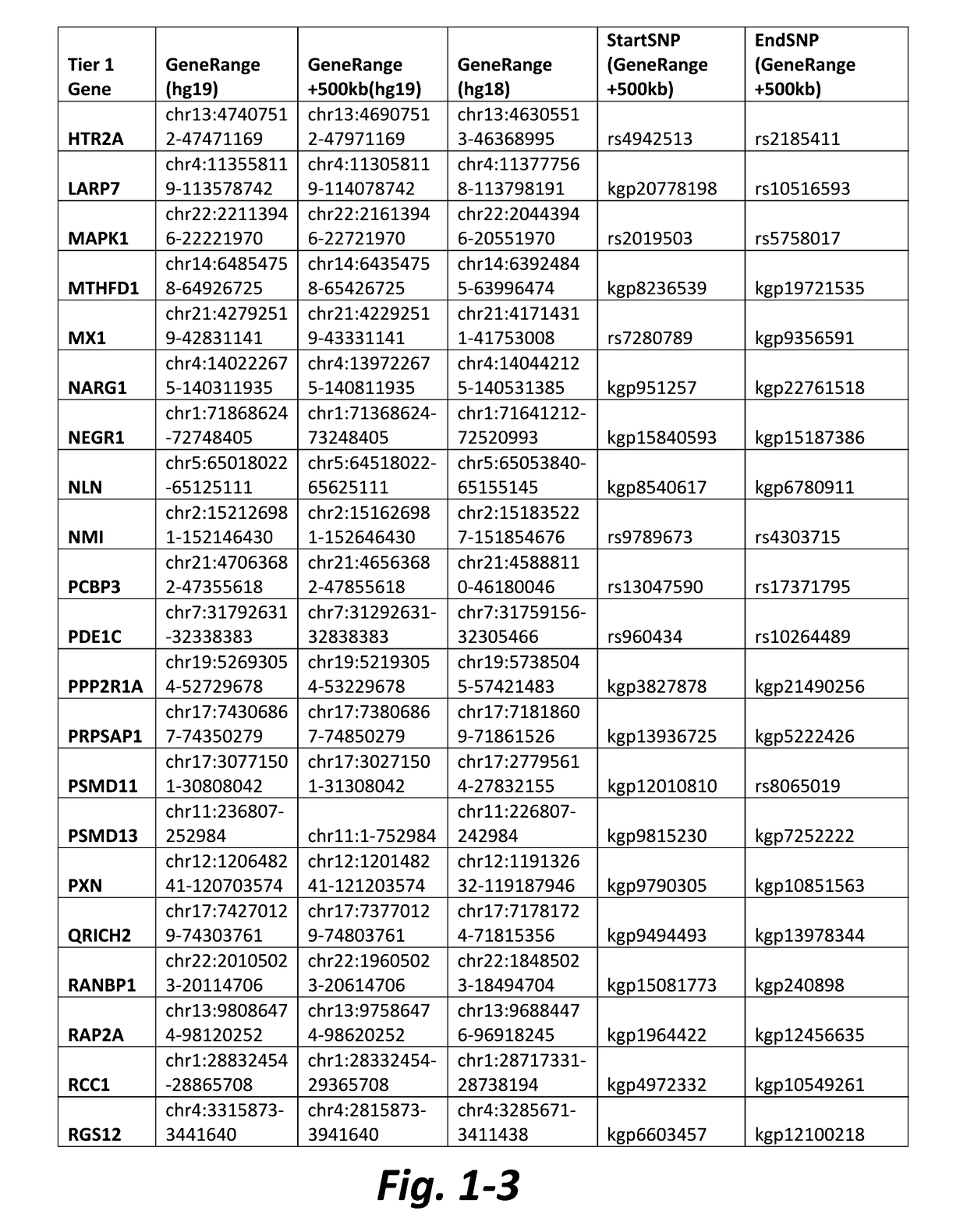
Multimarker panel based on PIGF for diabetes type 1 and 2
InactiveUS20060008829A1Peptide/protein ingredientsAntipyreticBlood vesselCardiovascular Complication
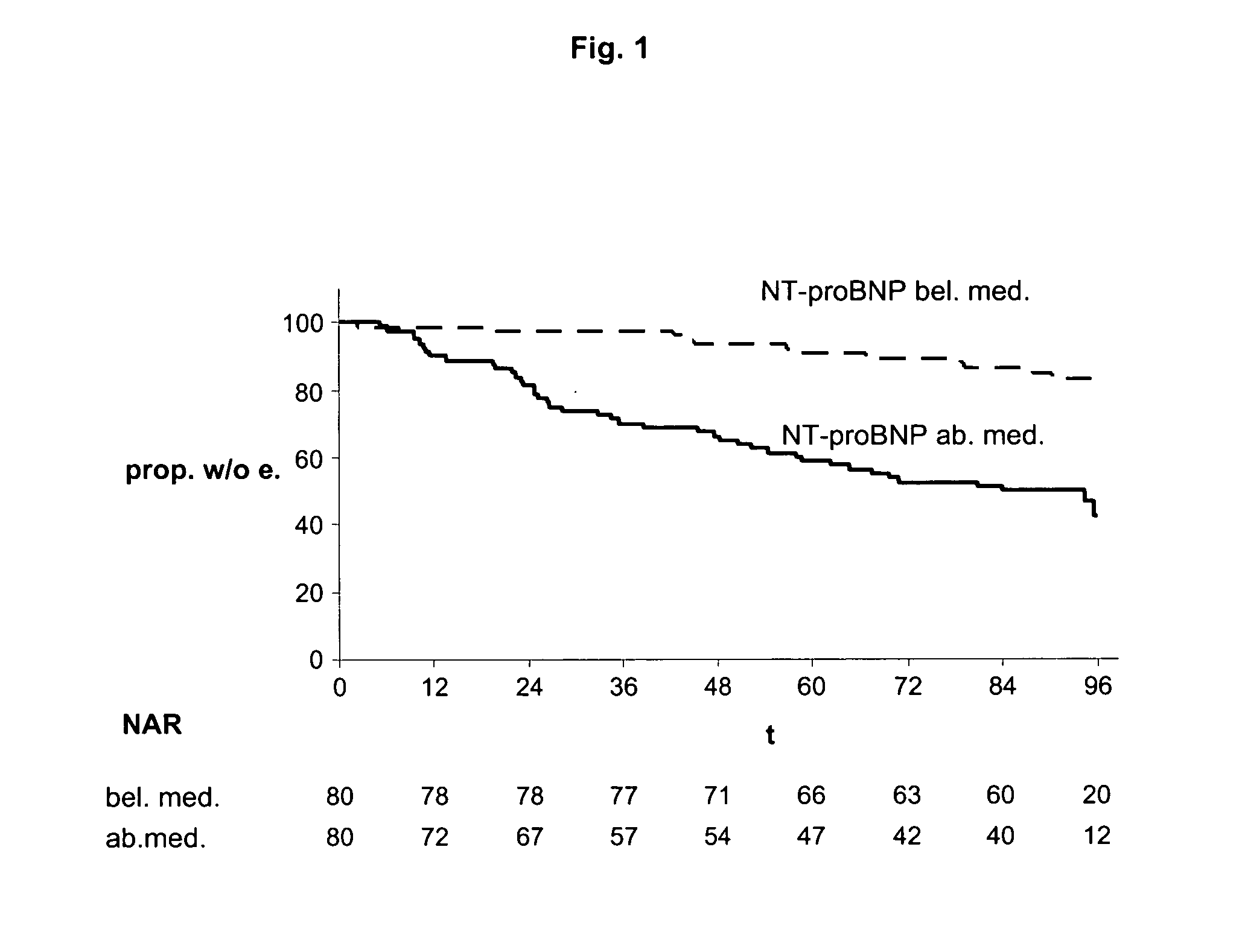
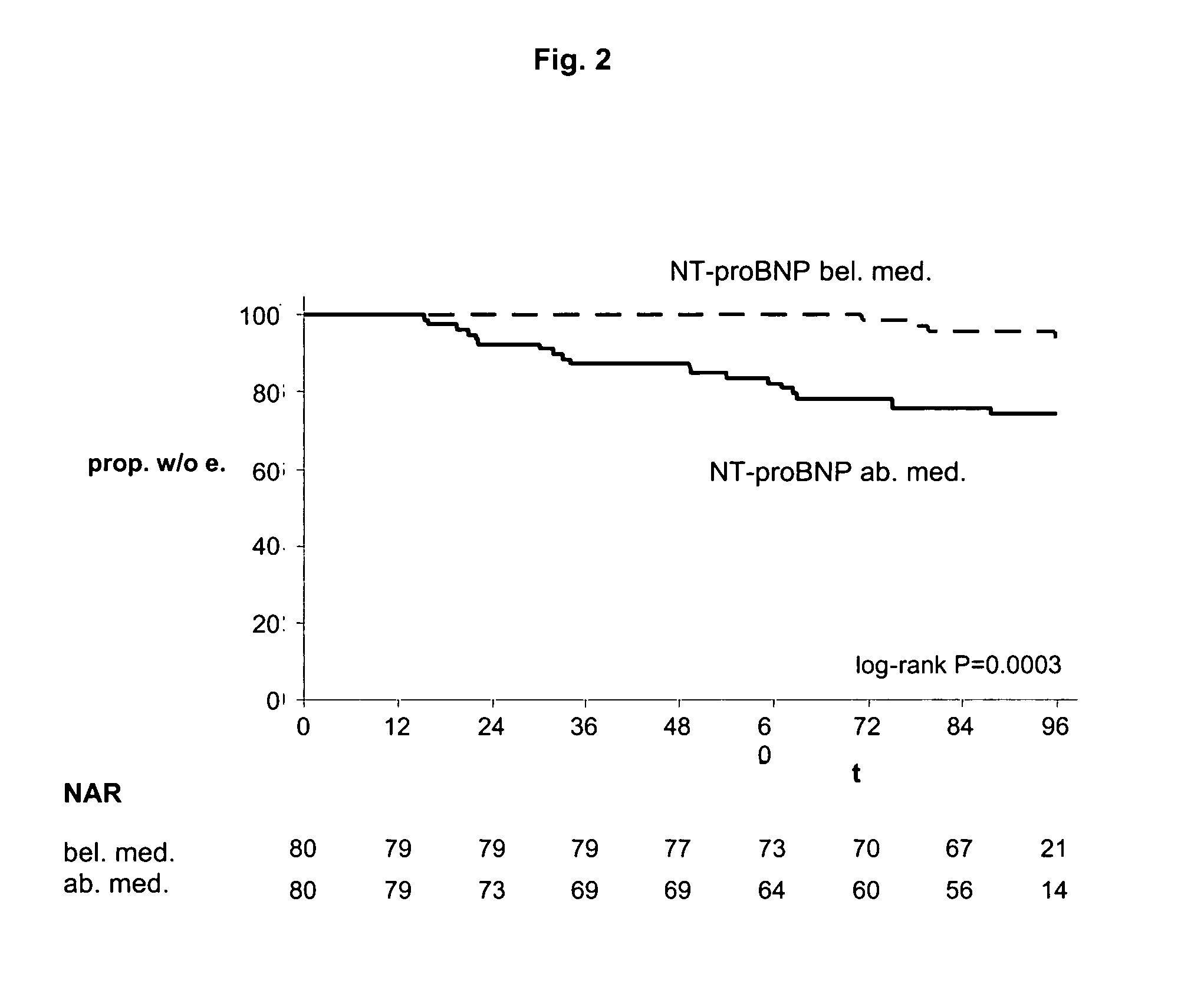
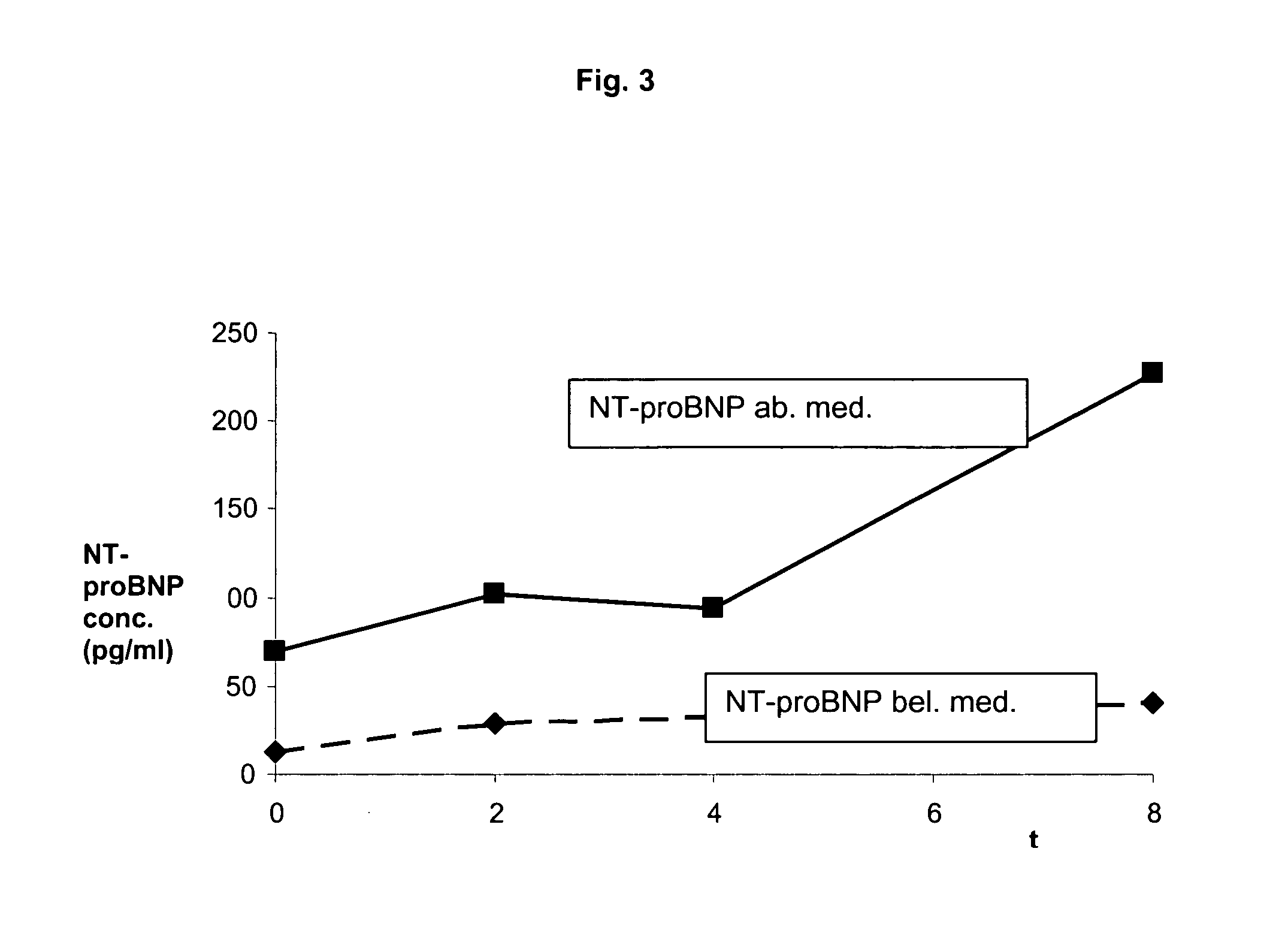
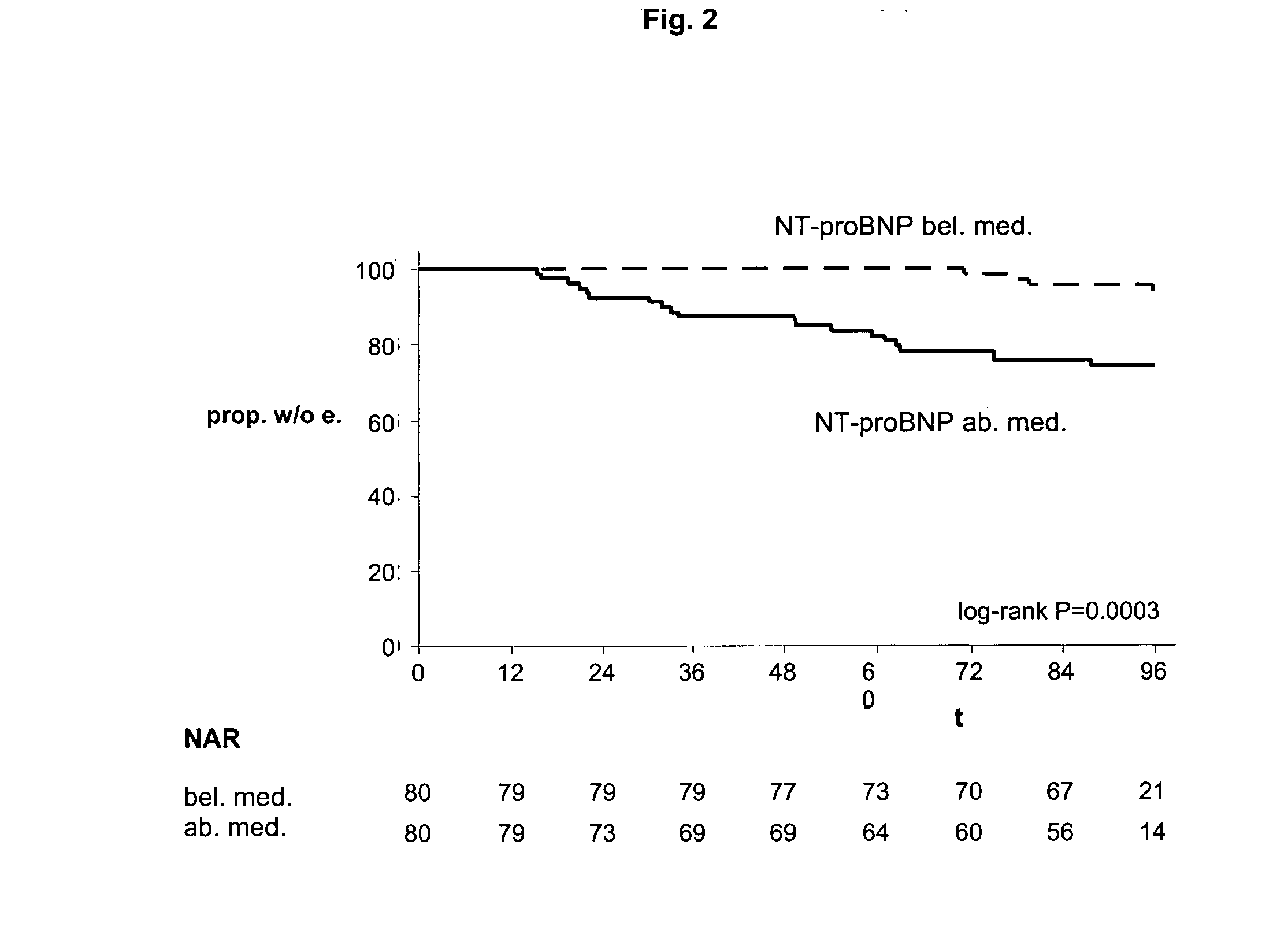
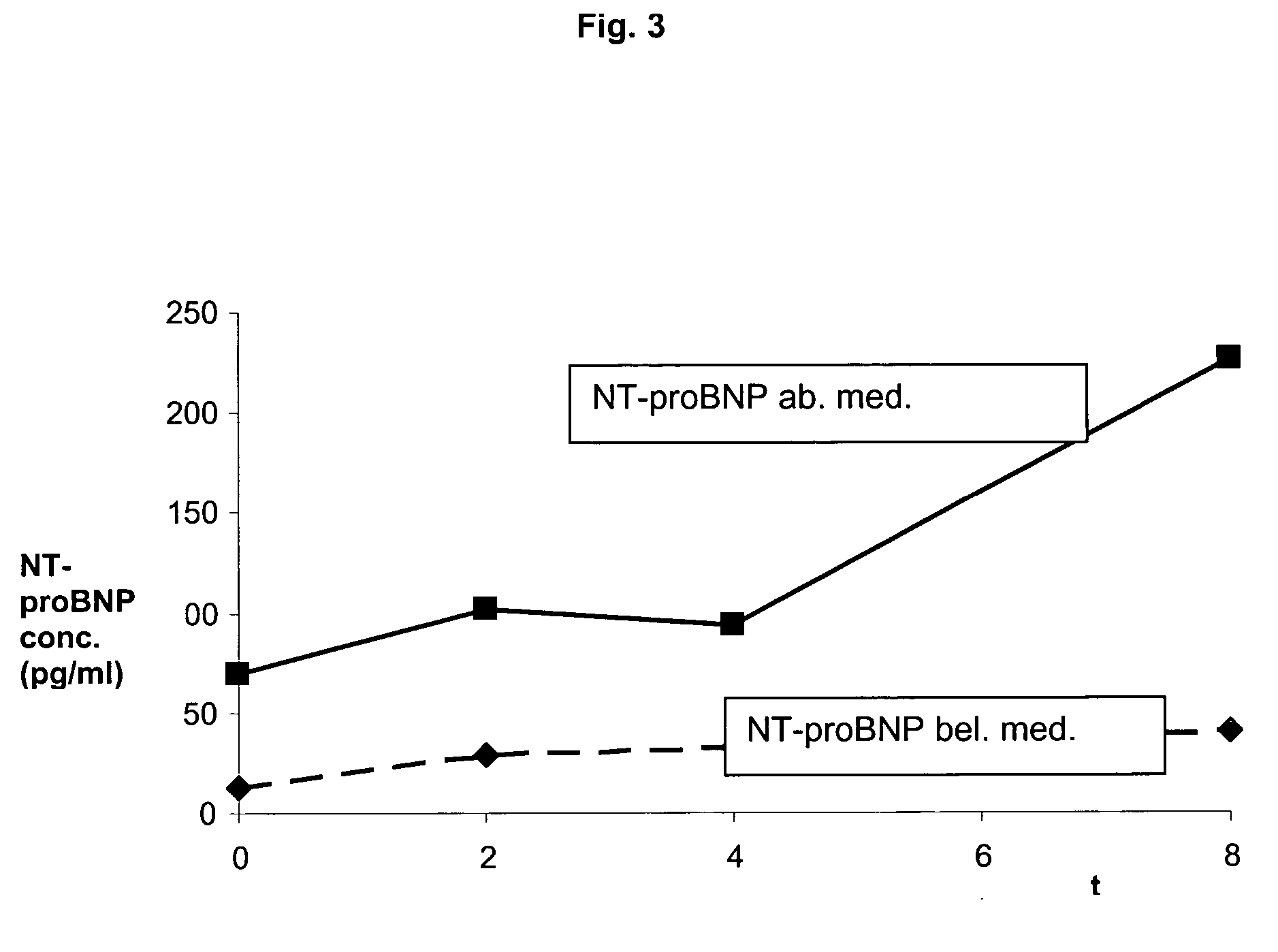
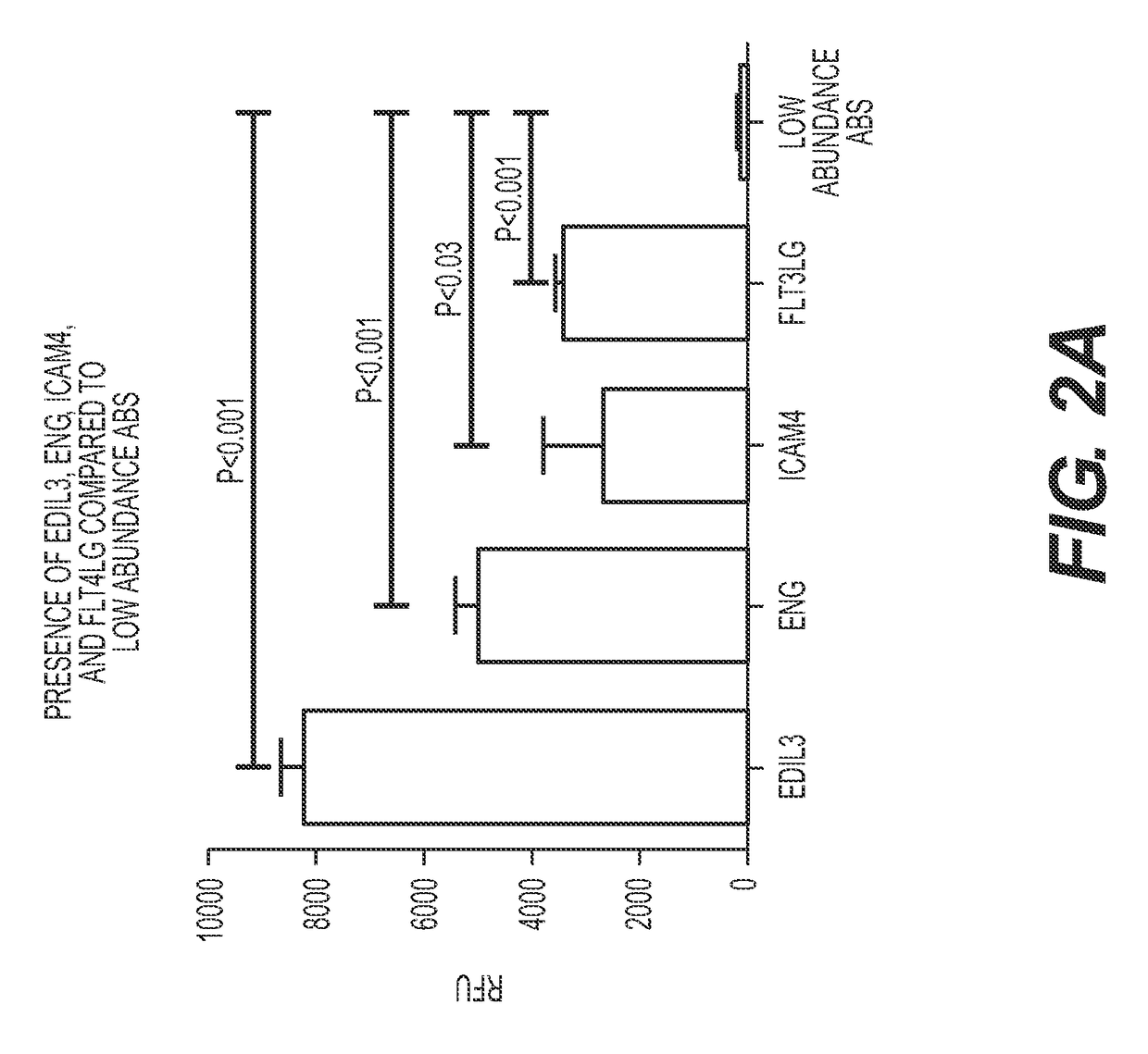
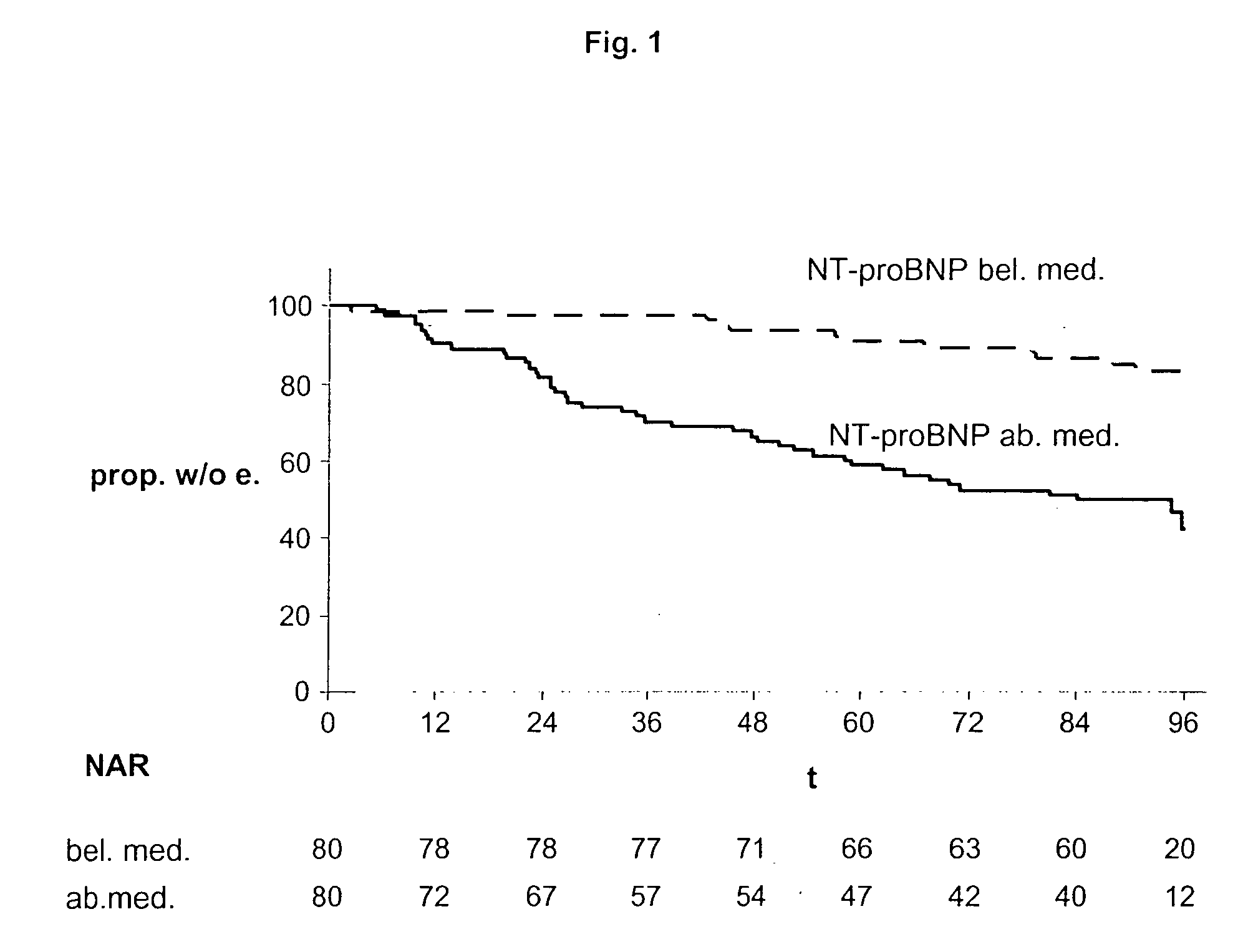
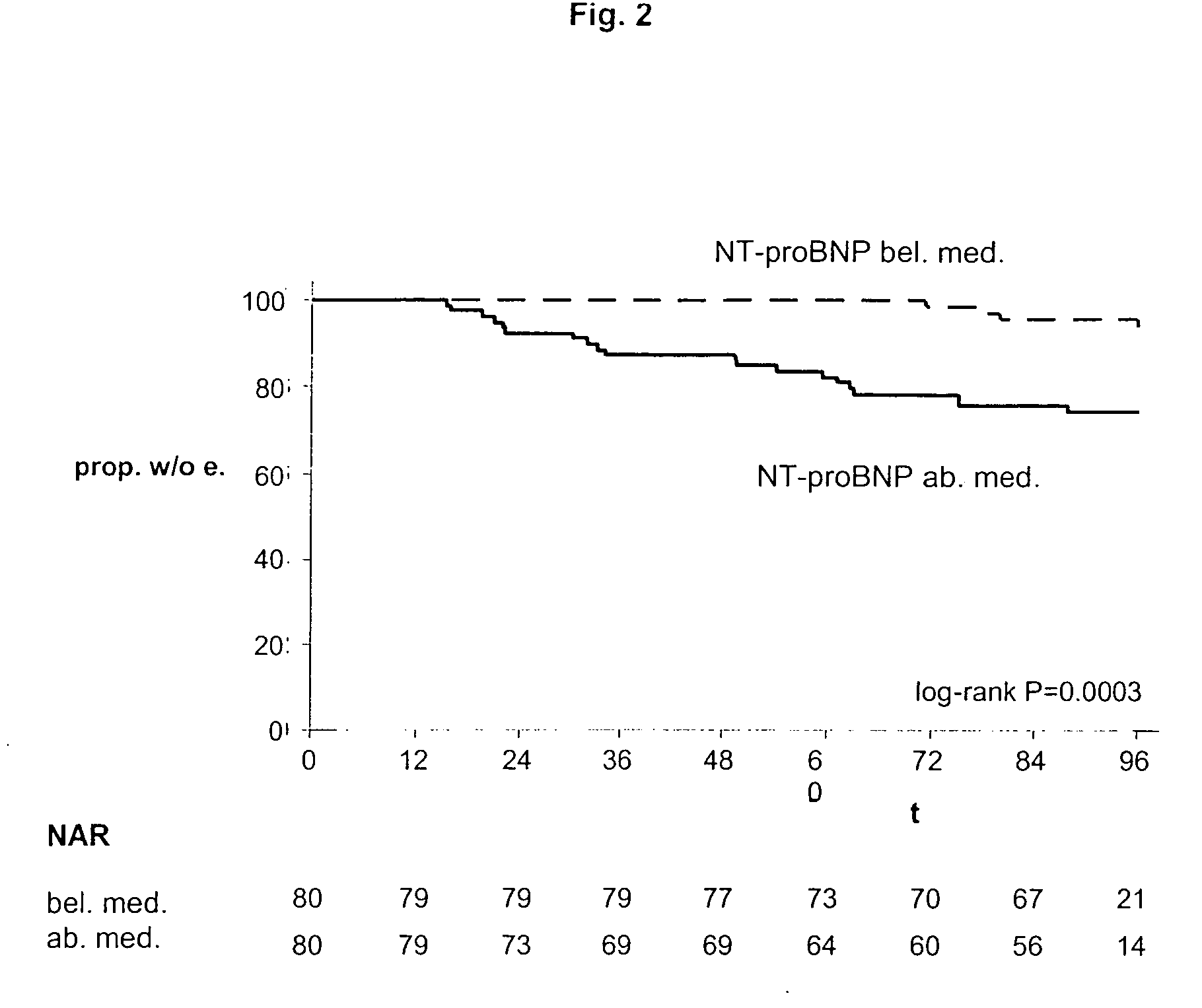
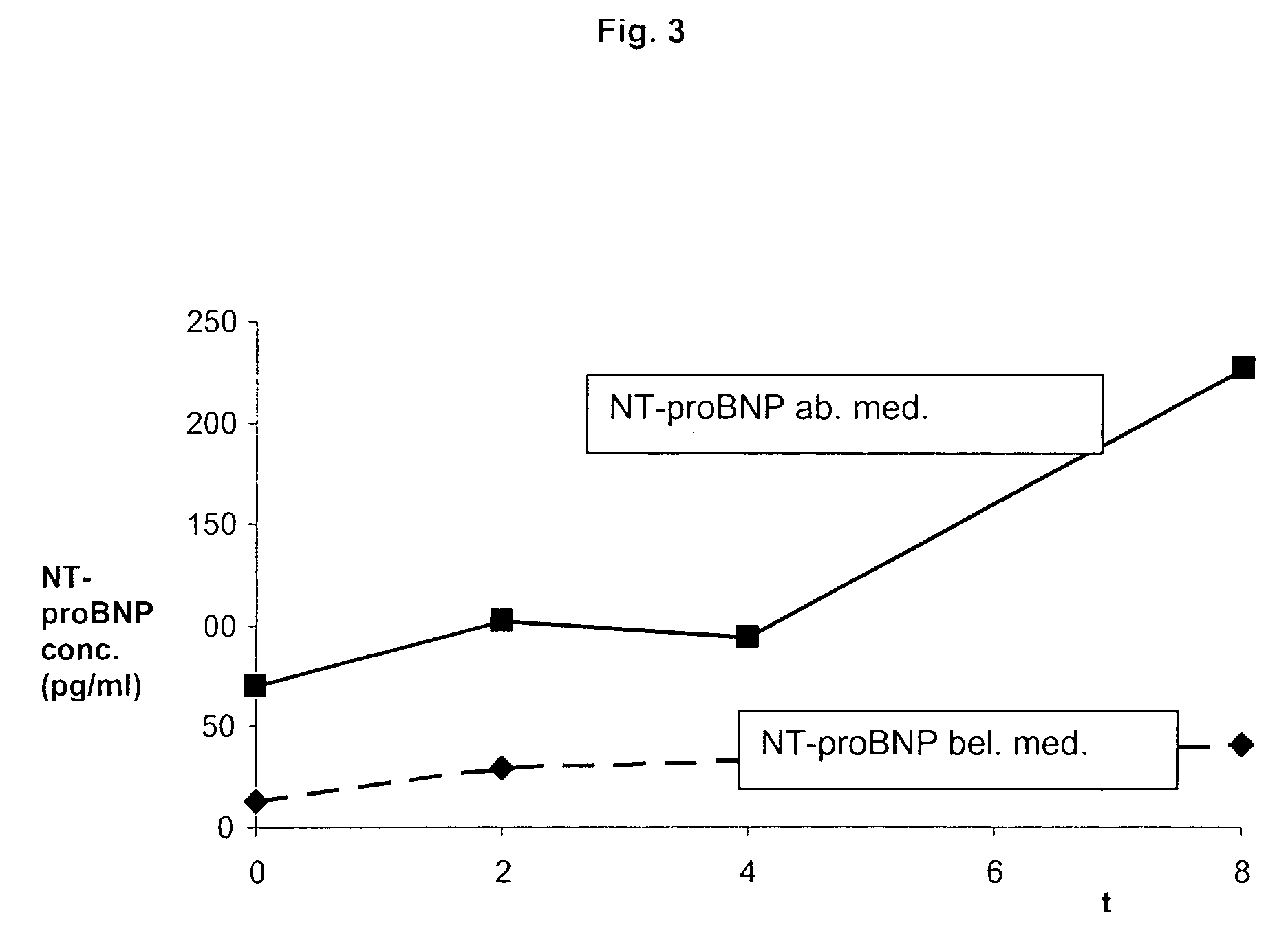
The present invention relates to a method and means for diagnosing or risk stratification of co-morbidities, particularly cardiovascular complications in diabetes patients. The invention particularly relates to a method for diagnosing whether a diabetes patient is suffering from a cardiovascular complication or is at risk of suffering from a cardiovascular complication, said method comprising the steps of (a) measuring, preferably in vitro, the level(s) of at least one cardiac hormone or a variant thereof in a sample from the patient, and (b) diagnosing the cardiovascular complication or the risk of suffering from cardiovascular complication by comparing the measured level(s) to known level(s) associated with the cardiovascular complication or risk. The present invention also relates to combining the measurement of markers comprising cardiac hormones, natriuretic peptides, inflammation-associated markers, angiogenesis markers, and markers for platelet activation. Preferred markers are brain natriuretic peptides (particularly NT-proBNP), PIGF, and sCD40L.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
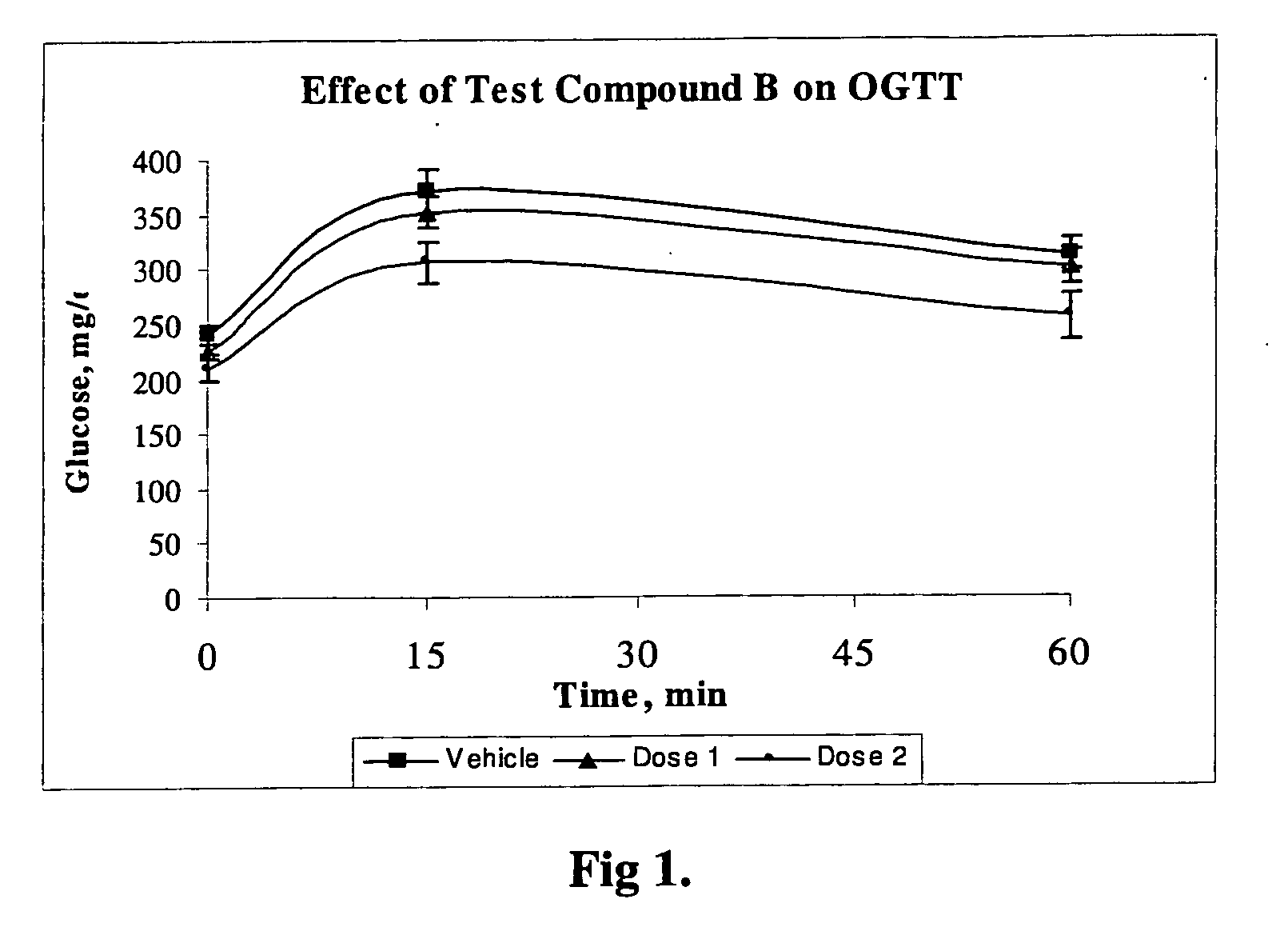
Compounds and their effects on feeding behaviour
The invention features peptides for the treatment or prevention of obesity, diabetes or co-morbidities of obesity; for reduction of appetite, food intake, calorie intake, body weight, or body weight gain; and for increase of energy expenditure in a subject.
Owner:IMPERIAL INNOVATIONS LTD
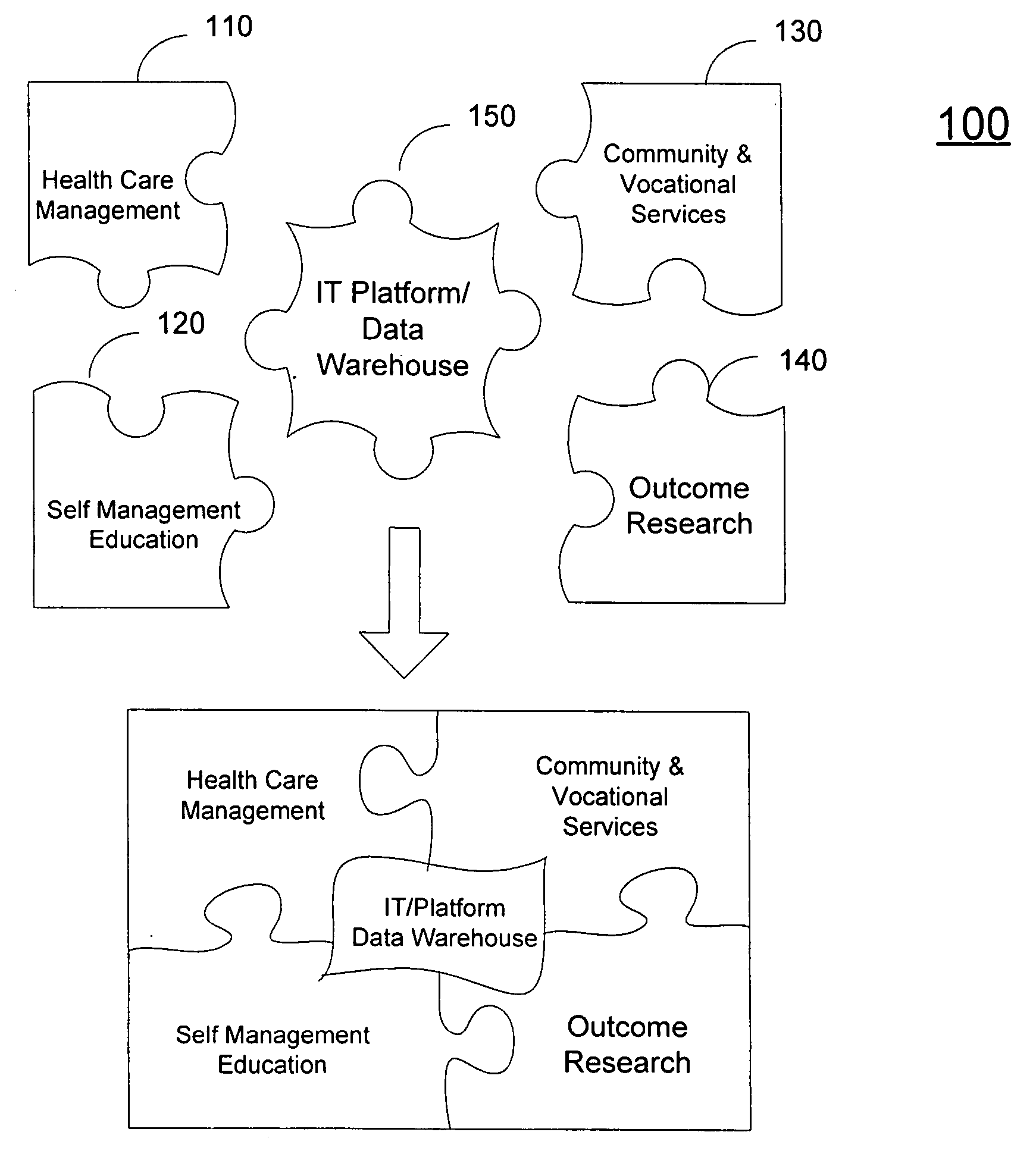
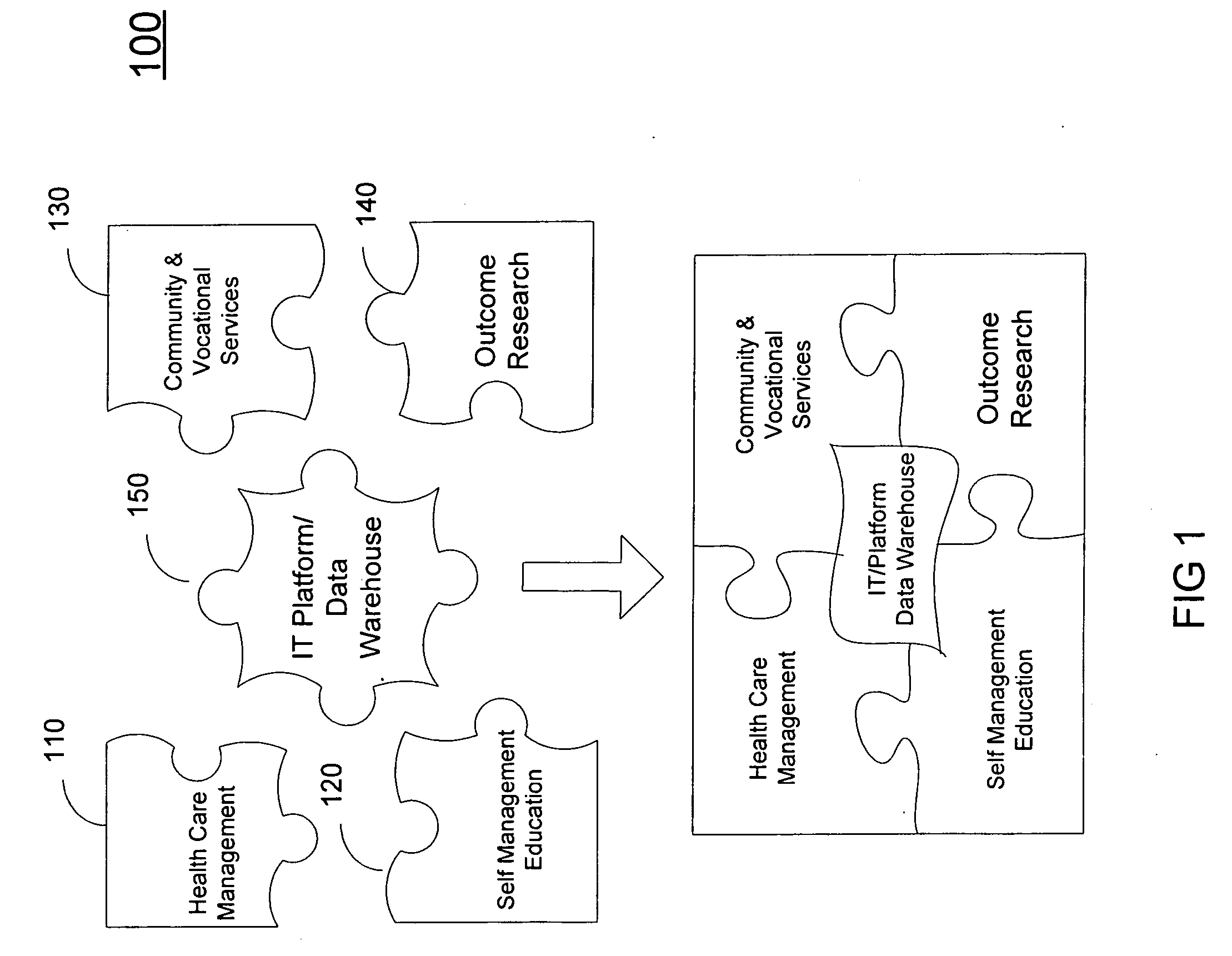
Coordinated health and human services delivery system and process
InactiveUS20060190303A1Good synergyGood for healthMedical communicationTherapiesCo morbidityBehavior modification
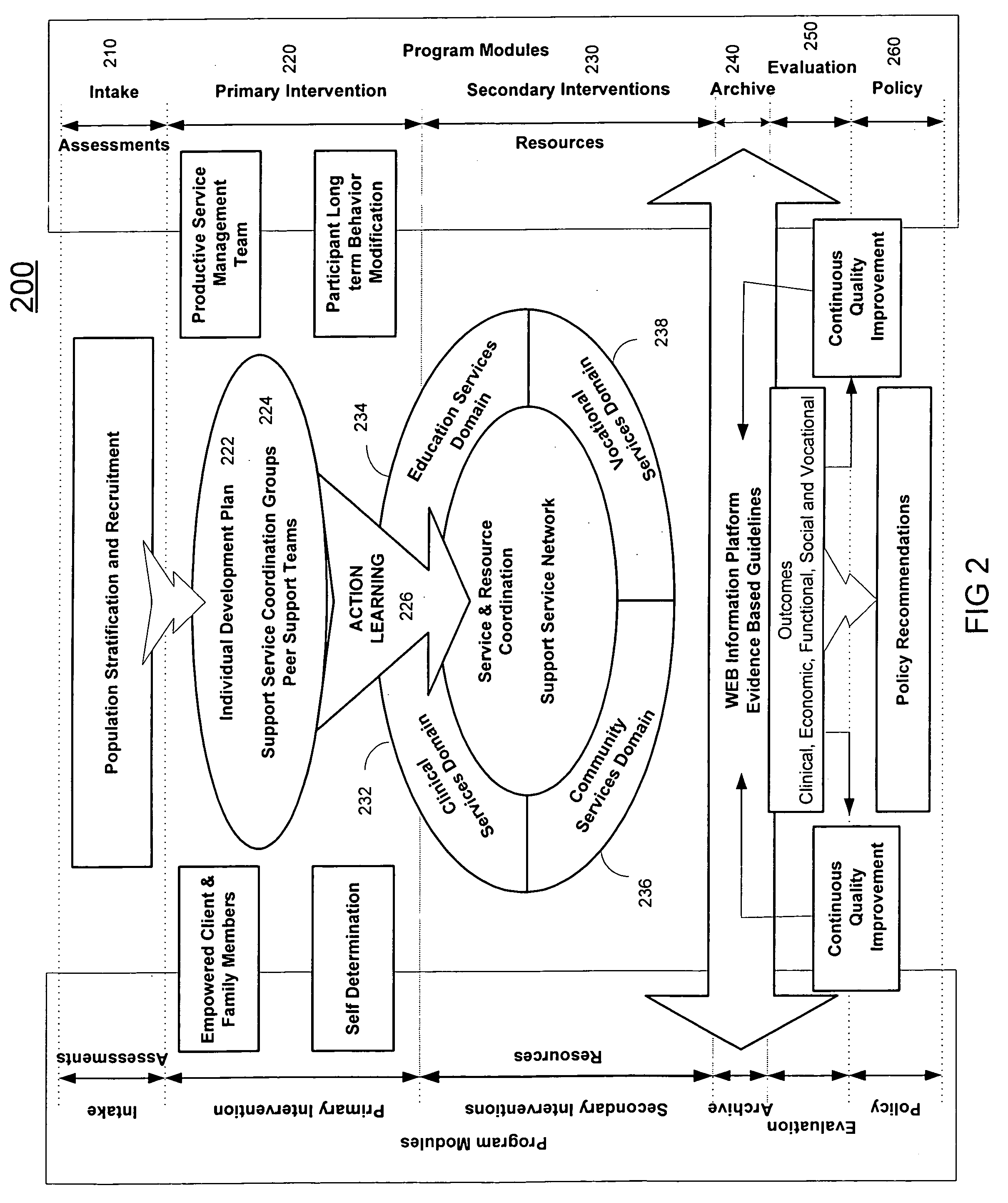
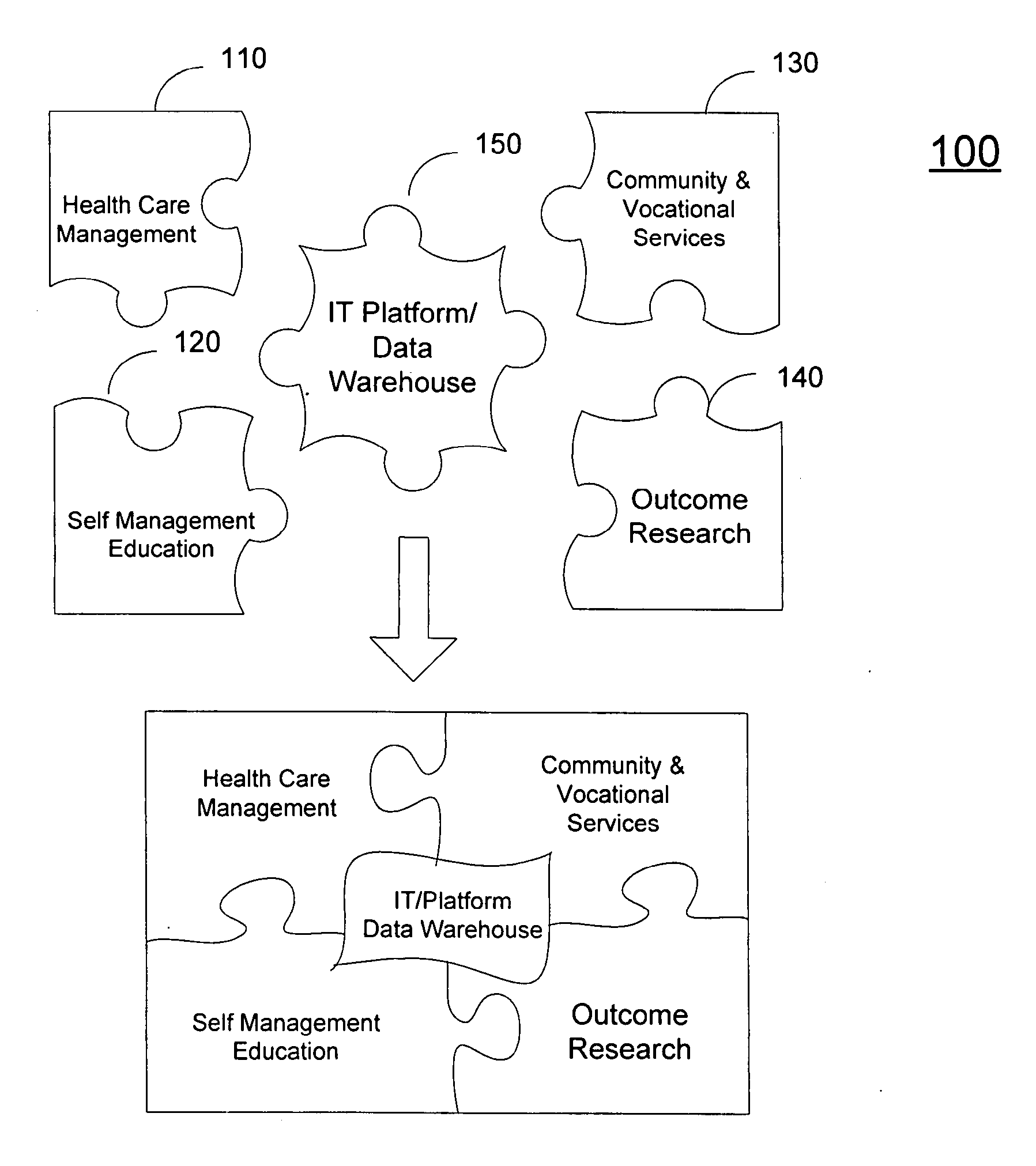
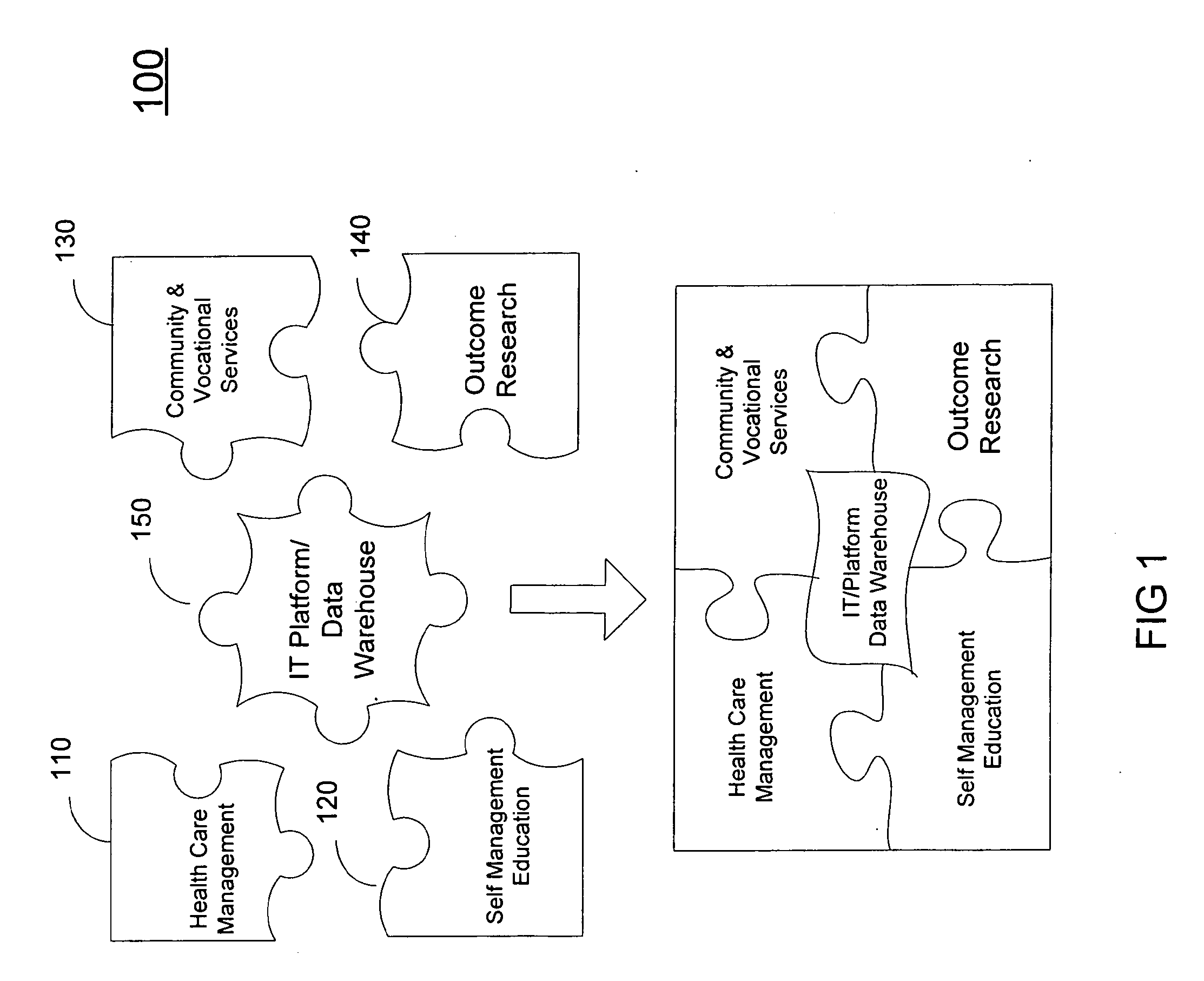
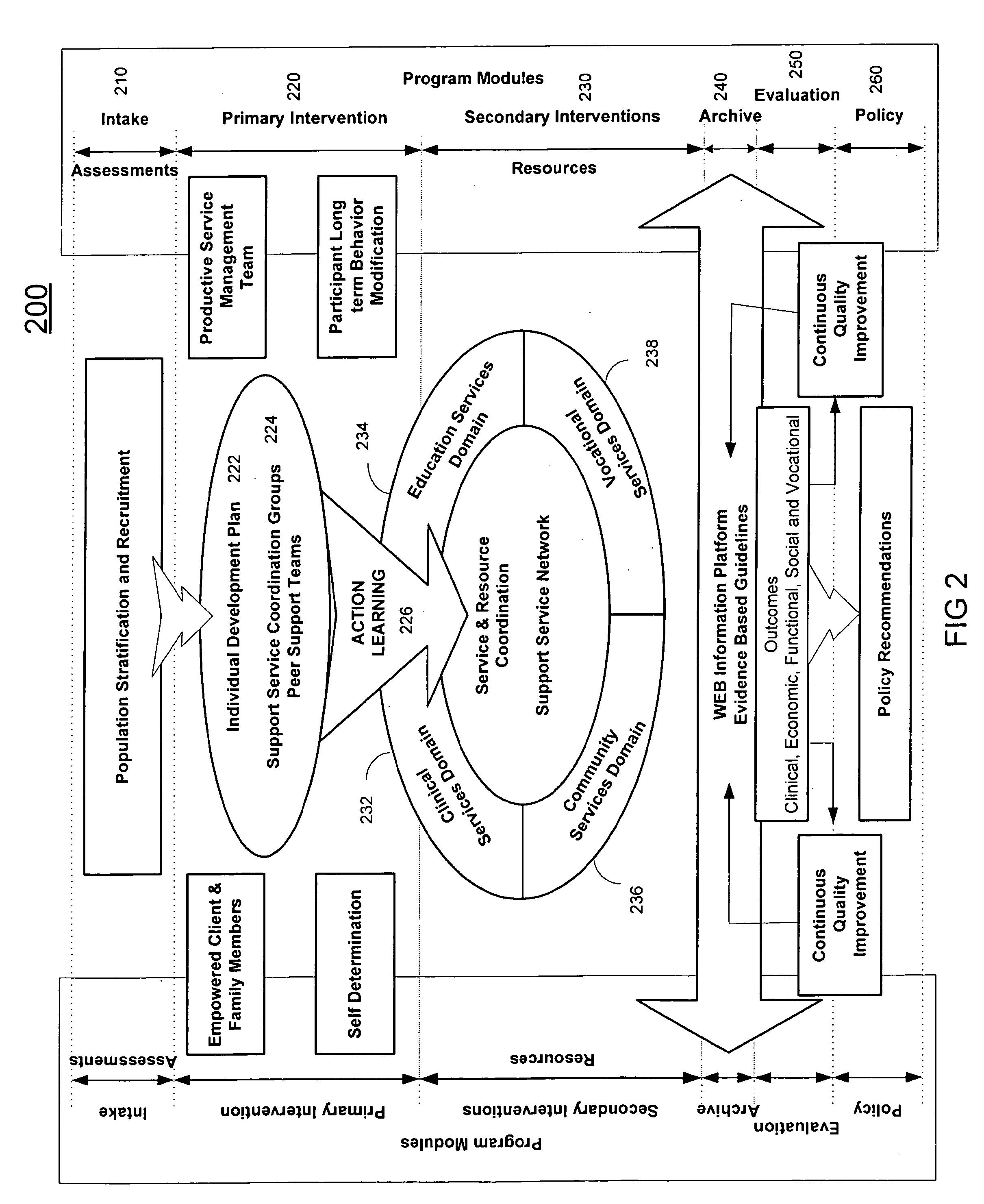
A system (200) and method (500) is provided for a coordinated health care service delivery program. The method can include providing services to clients at high risk for chronic disease including co-morbidities and consequent disabilities associated with the chronic disease, linking community and vocational services (130) for facilitating community inclusion to supplement fundamental clinical and economic goals, creating a comprehensive and dynamic individual development plan (222) to involve the client and family members as active program team members for stressing client-centric collaborative goal setting, and applying action learning (226) to promote behavior modification and lifestyle change.
Owner:THE TRINITY MANAGEMENT GROUP
Combination treatment of specific forms of epilepsy
InactiveUS20170071949A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Novel Compounds
ActiveUS20100168110A1Improve insulin resistancePreventing and delaying progressionBiocideNervous disorderDyslipidemiaCo morbidity
The present invention discloses a novel thyroid like compounds of formula (I), wherein R1 R2, R3, R4 and Z are as defined in the specification, method for its preparation, composition containing such compounds and use of such compounds and composition as medicament. Further, compounds of formula (I) has significantly low binding affinity to thyroid receptors and thus considerably devoid of thyrotoxic effects. The invention also relates to the use of the compound of formula (I) for the preparation of a medicament for treating various disease conditions such as obesity, dyslipidemia, metabolic syndrome and co-morbidities associated with metabolic syndrome.
Owner:TORRENT PHARMA LTD
Multimarker panel for diabetes type 1 and 2
The present invention relates to a method and means for diagnosing or risk stratification of co-morbidities, particularly cardiovascular complications in diabetes patients. The invention particularly relates to a method for diagnosing whether a diabetes patient is suffering from a cardiovascular complication or is at risk of suffering from a cardiovascular complication, said method comprising the steps of (a) measuring, preferably in vitro, the level(s) of at least one cardiac hormone or a variant thereof in a sample from the patient, and (b) diagnosing the cardiovascular complication or the risk of suffering from cardiovascular complication by comparing the measured level(s) to known level(s) associated with the cardiovascular complication or risk. The present invention also relates to combining the measurement of markers comprising cardiac hormones, natriuretic peptides, inflammation-associated markers, angiogenesis markers, and markers for platelet activation. Preferred markers are brain natriuretic peptides (particularly NT-proBNP), PIGF, and sCD40L.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
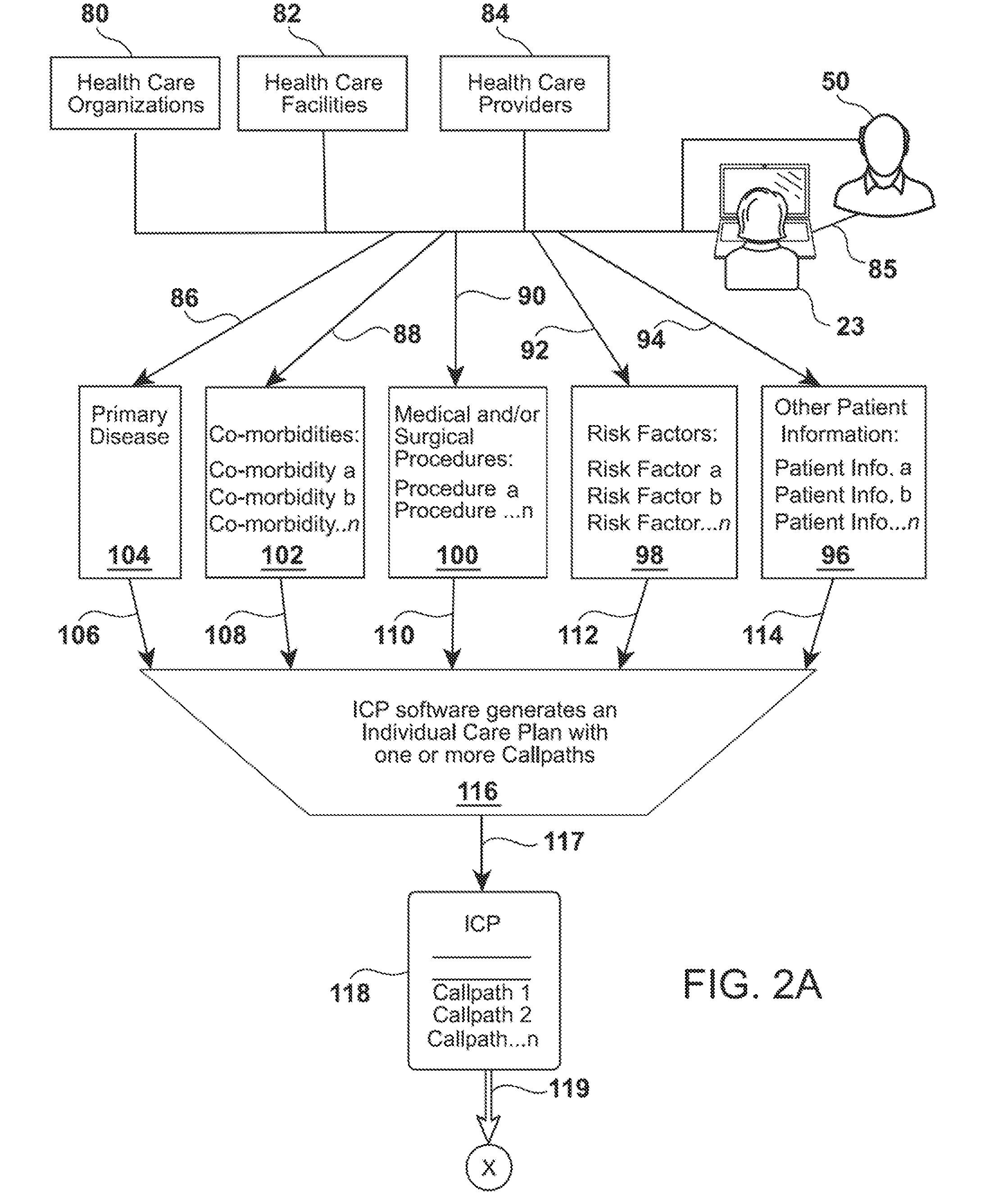
Medical Transitional Care Patient Management System and Associated Business Method
InactiveUS20150112721A1Data processing applicationsHealth-index calculationPersonal carePersonalization
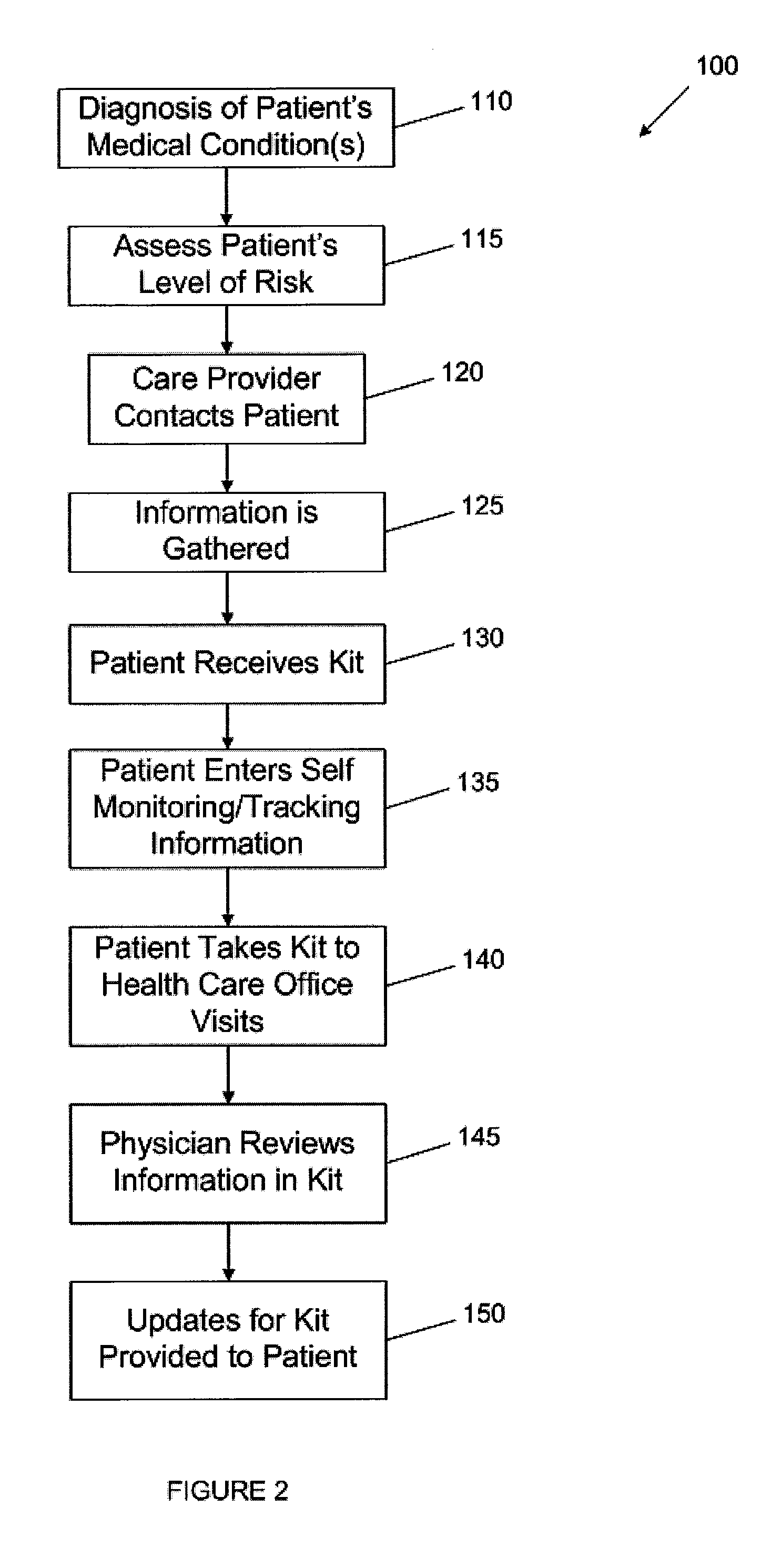
The present invention pertains to novel medical post-discharge patient management system and associated business method that provides a means to track patients health after post-discharge using a program that employees a plurality of mathematical algorithms and software instructions for analyzing data that results in developing customized individual care plans that incorporate all of the unique co-morbidities of each patient. The system and software components includes an individual care plan (“ICP”) engine which receives data regarding the patient, their contact information and co-morbidities and uses the data to construct custom individualized care plans for each patient, based on their unique case mix of morbidities. The system software also includes IVR engine the works in conjunction with the ICP engine to contact patients using outbound interactive voice response, email and text messaging to ask the questions in the patients' CallPaths.
Owner:BLOODSWORTH JR JPHN OGDEN
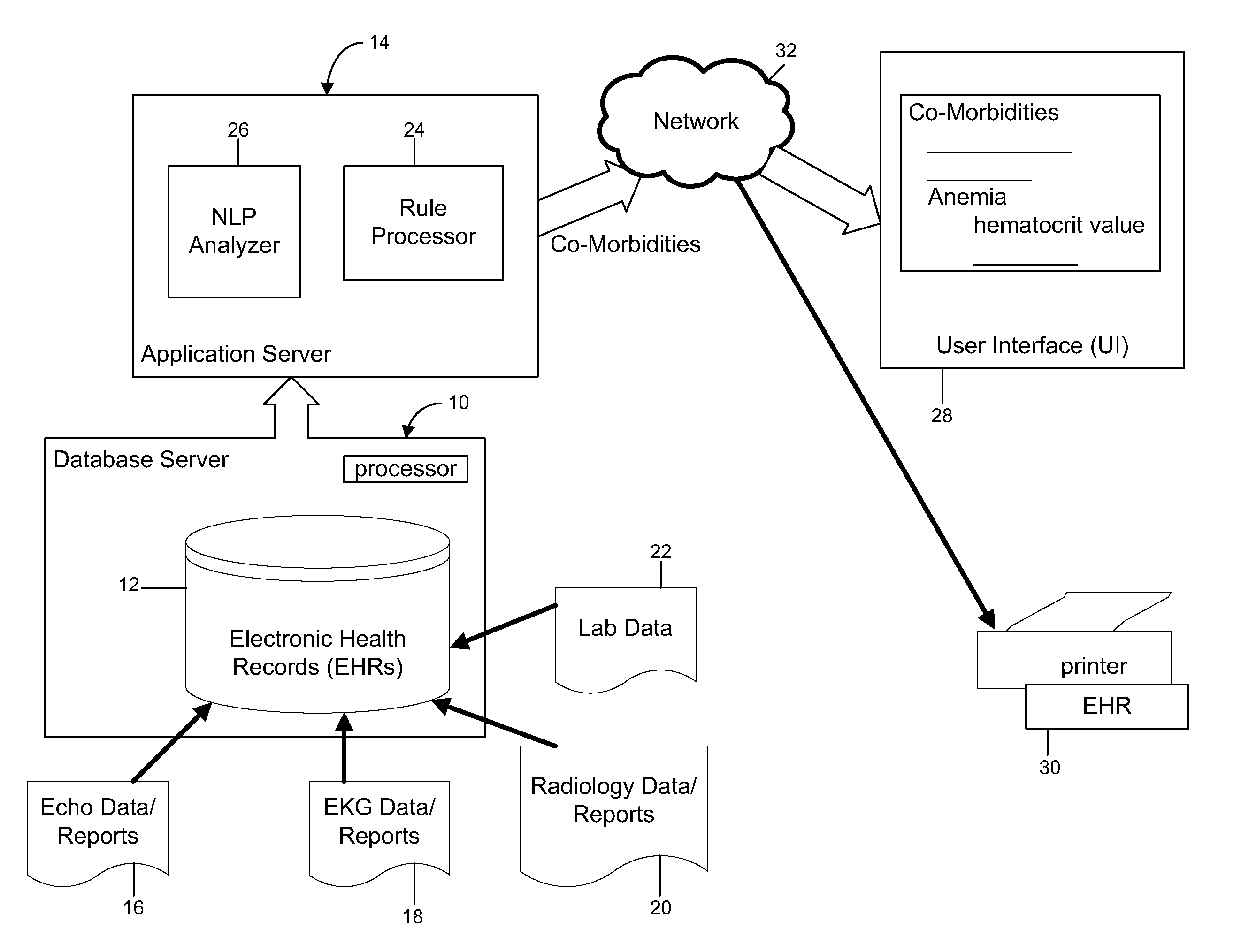
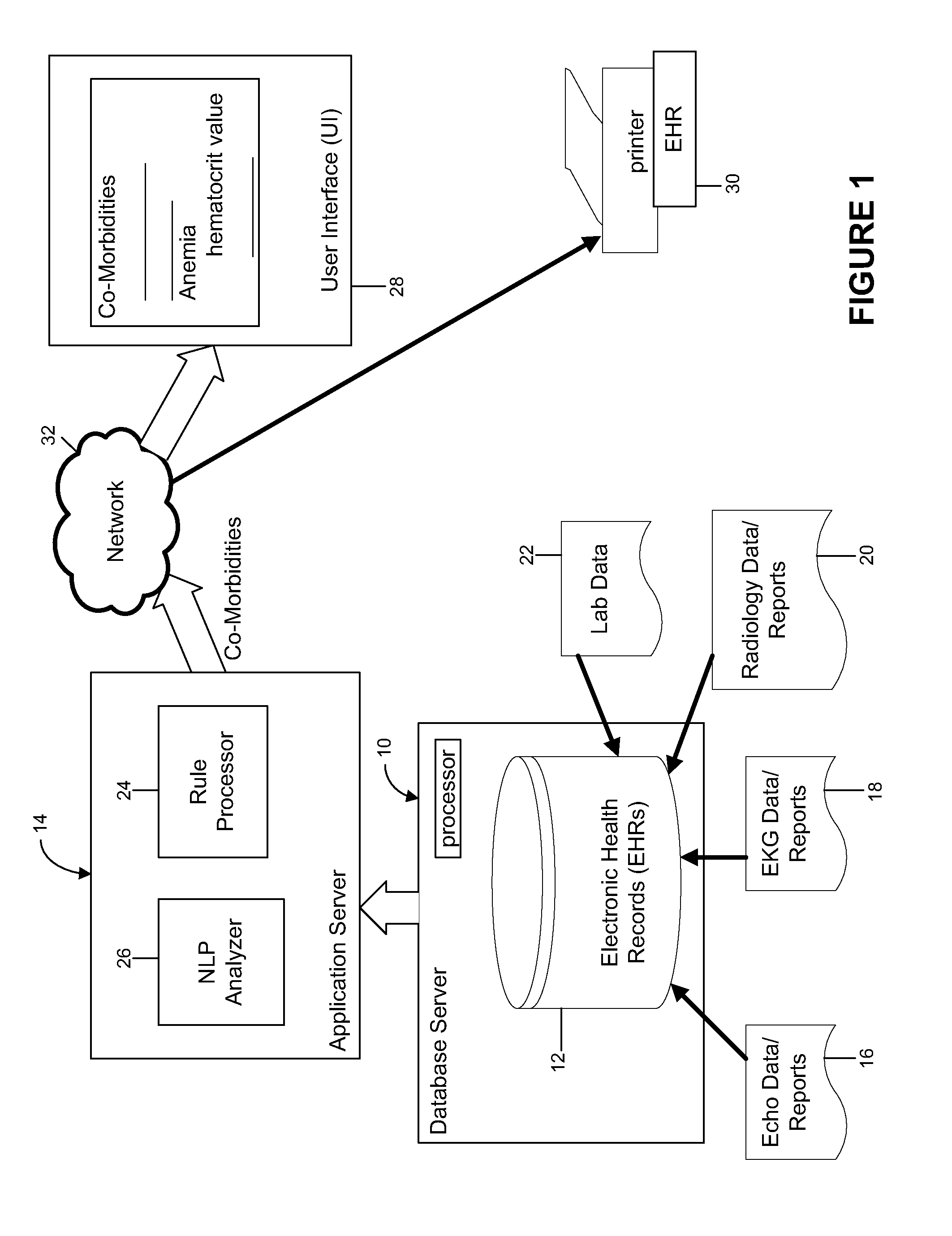
Automated identification and documentation of co-morbidities from patients electronic health record in the emergency room
InactiveUS20140067424A1Enhances patient careAccurate recordPatient personal data managementOffice automationCo morbidityDocumentation procedure
An inventive system and method for identifying and documenting co-morbidities is provided. The method can include selecting co-morbidity-related clinical data in accordance with one or more rules, said clinical data selected for a patient from the history data of the patient, pushing the selected clinical data to a display on a display device, displaying the selected clinical data on the display device along with the one or more rules, analyzing the displayed selected clinical data in accordance with the displayed one or more rules, validating the displayed selected data and storing the validated data. In one aspect, the method can further comprise sending the validated data to billing and / or printing the validated data. In one aspect, the clinical data can comprise one or more of laboratory data, EKG data, Echo data and radiology data.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Combination treatment of specific forms of epilepsy
InactiveUS20180325909A1Reduce seizuresEliminate side effectsAnhydride/acid/halide active ingredientsHeterocyclic compound active ingredientsSide effectCo morbidity
Formulations for and methods of treatment of Dravet syndrome that avoid side effects are disclosed. The formulations comprise a 5-HT receptor agonists which does not agonize selected 5-HT receptor subtypes, and in particular does not agonize the receptor subtype 5-HT2B. Also disclosed are combinations of such 5-HT receptor agonists. Also disclosed are combinations of such 5-HT receptor agonists and SSRIs, SNRIs, and triptans for treating co-morbidities associated with Dravet syndrome.
Owner:ZOGENIX INT
Personalized health management tool
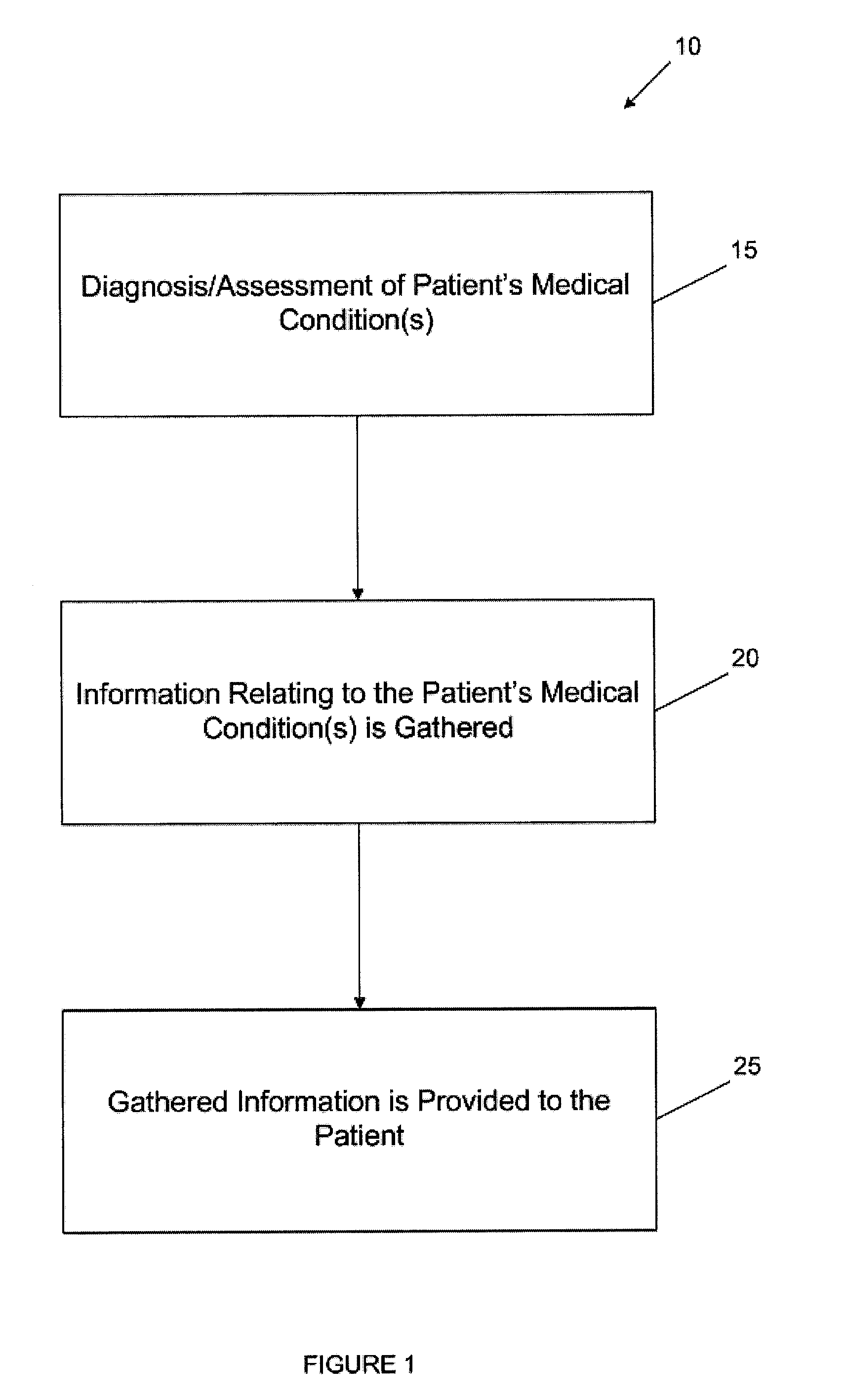
A personalized health management tool provides personalized health management information to one or more patients or members of a health plan for use both by the patient and the patient's healthcare provider(s) to improve the quality and efficiency of interactions between the patient and the provider(s). The personalized health management tool includes information relating to a patient's medical conditions and may include integrated guidelines that provide guidance on management and treatment of co-morbidities of the patient. The tool further includes information relating to the self-management of the patient's condition(s), information relating to the medical history of a patient, and / or information relating to the recommended care and treatment required for the patient's condition(s). For example, the health management tool may include EBM guidelines regarding the medical condition(s), integrated guidelines for multiple condition management, and / or information relating to resources related to the patient's medical condition(s) that are available to the patient for assistance with healthcare, financial issues, and / or social issues. A method for providing personalized health management information to one or more patients or members of a health plan includes identifying patients or members having one or more specific medical conditions; gathering personalized health data for each identified patient or member; and providing each identified patient or member with a personalized health management tool including information on the specific medical conditions, self-management of the specific medical conditions, treatment of the medical conditions for use by the patient's healthcare providers, and the personalized health data gathered for the respective identified patient or member.
Owner:OVATIONS
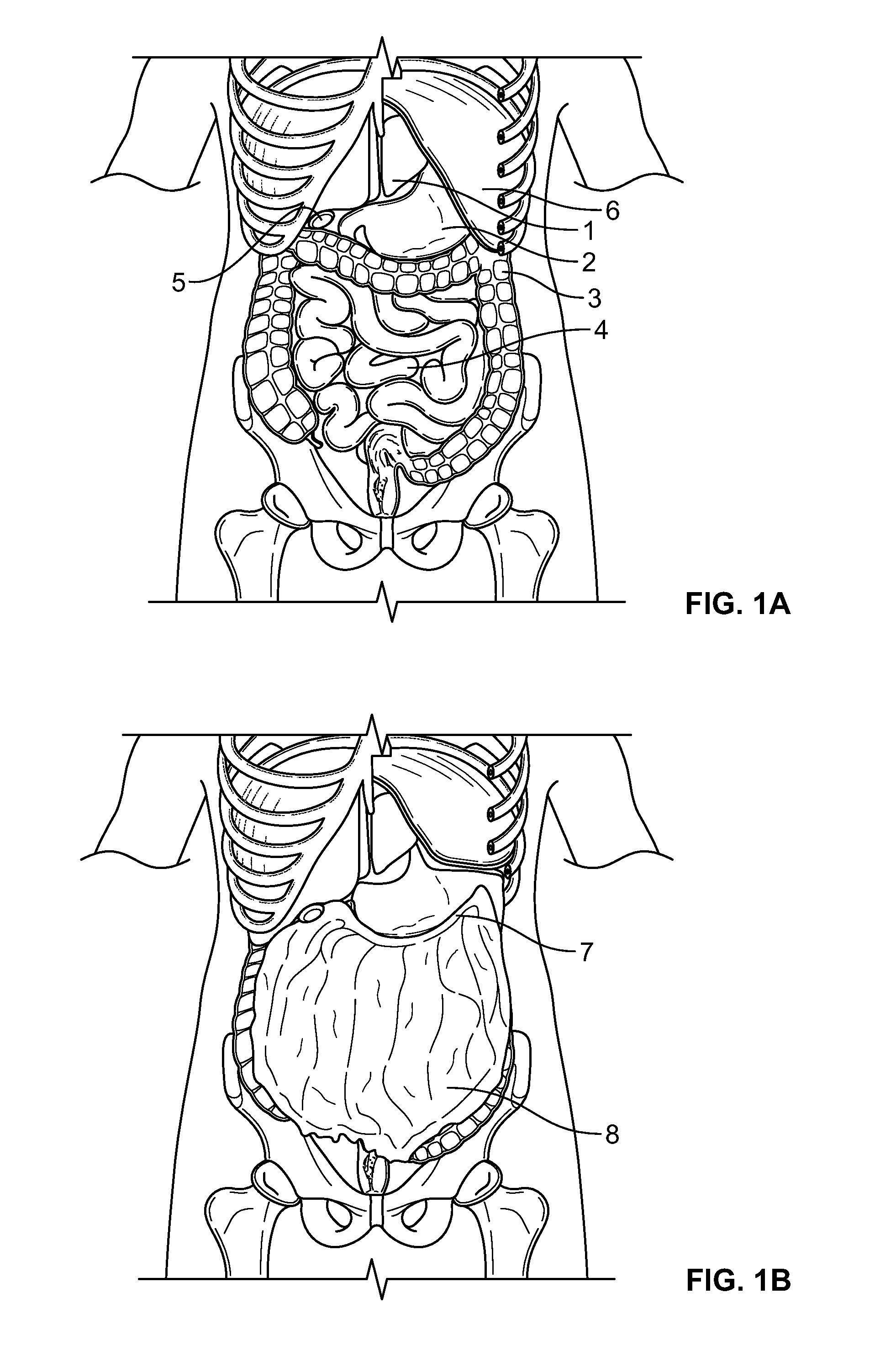
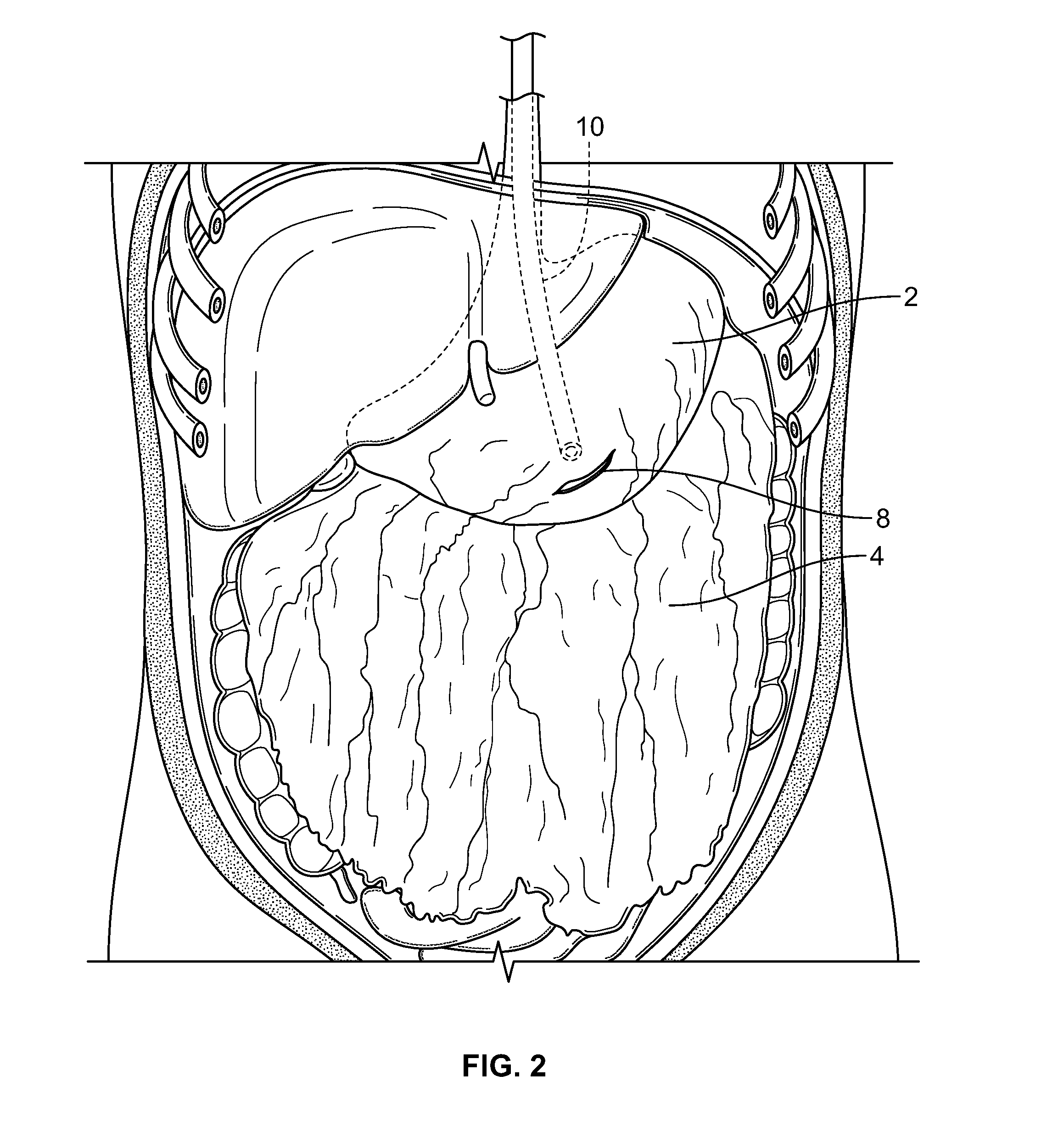
Methods and devices for removing omental tissue
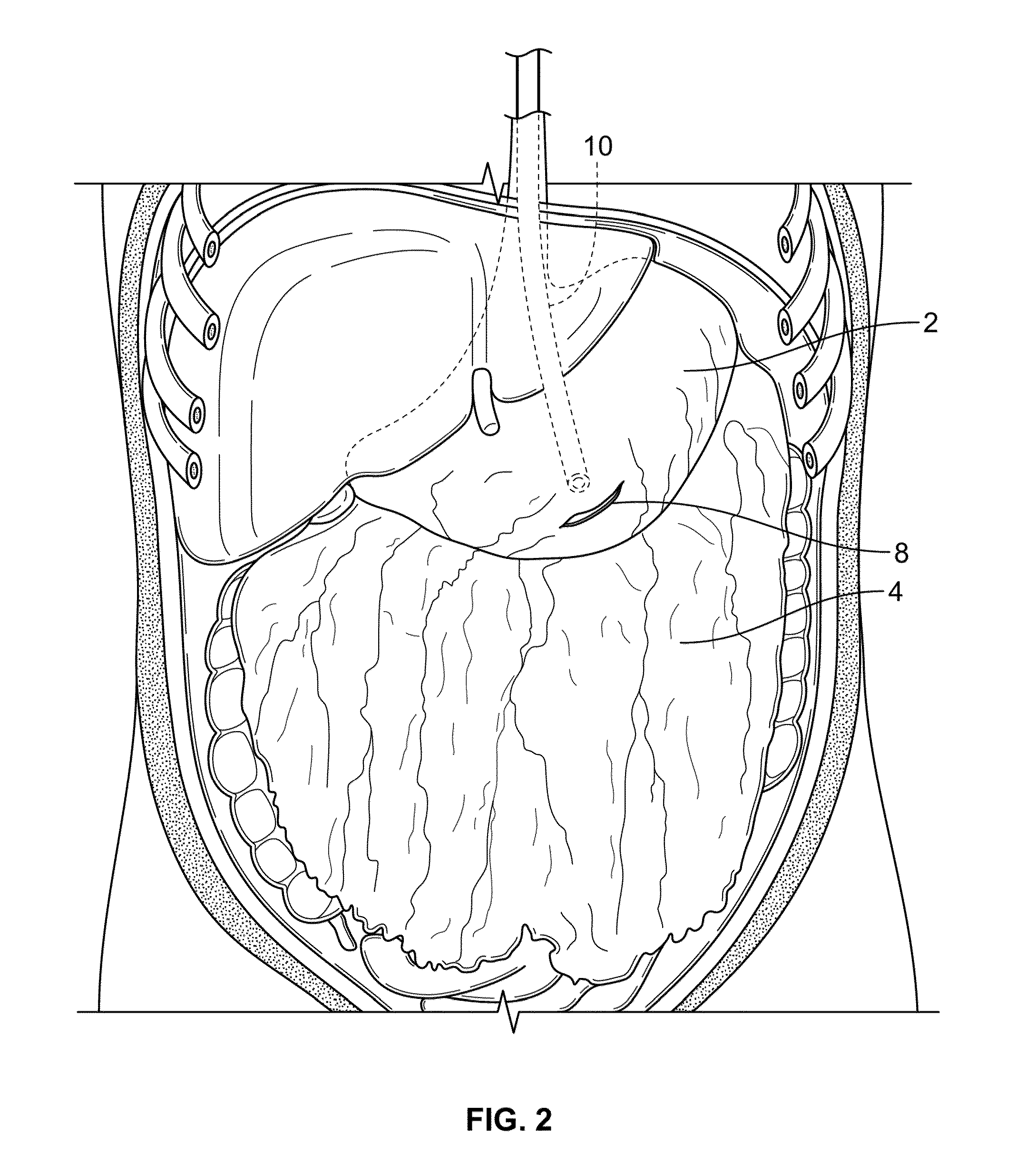
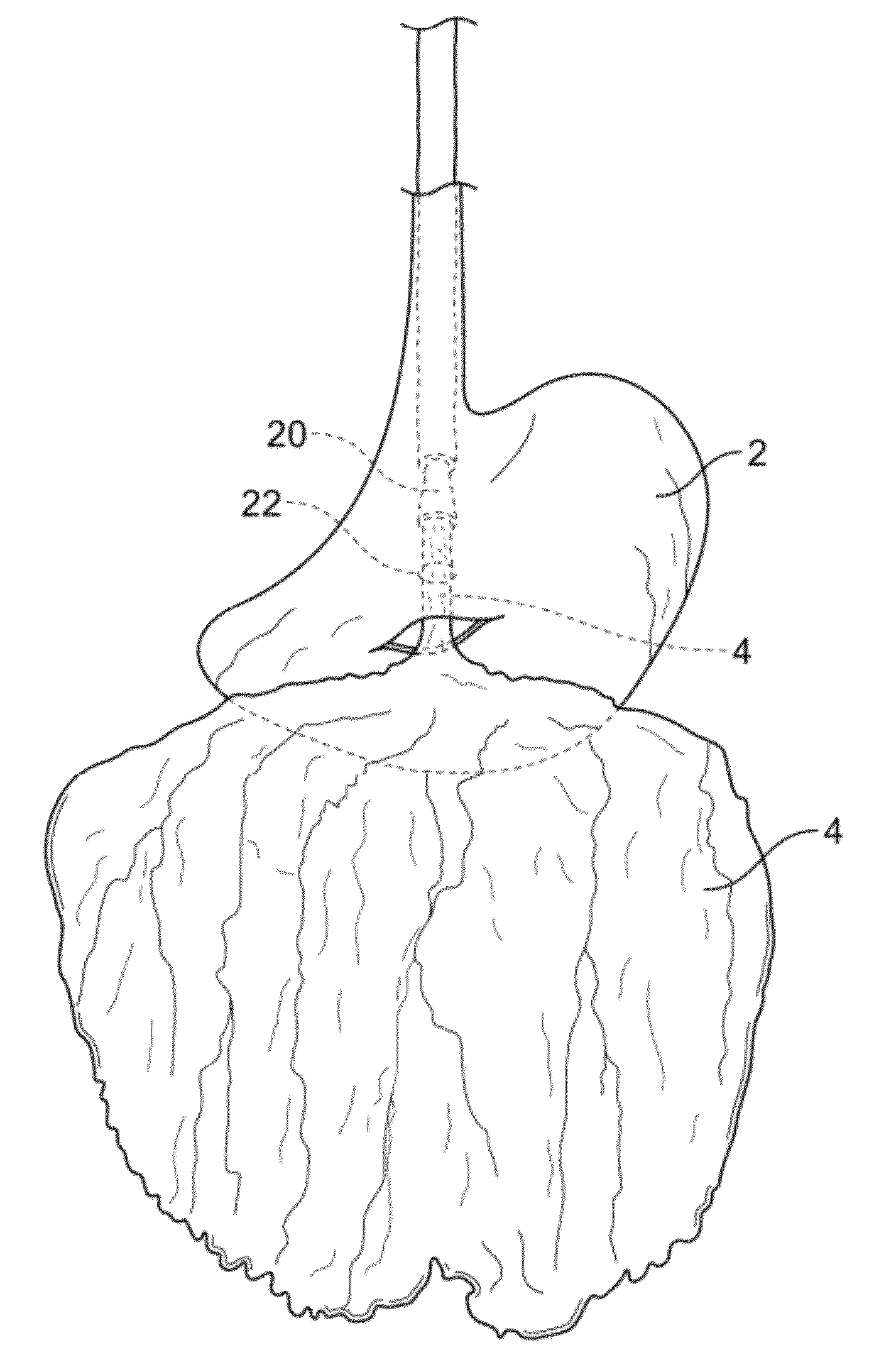
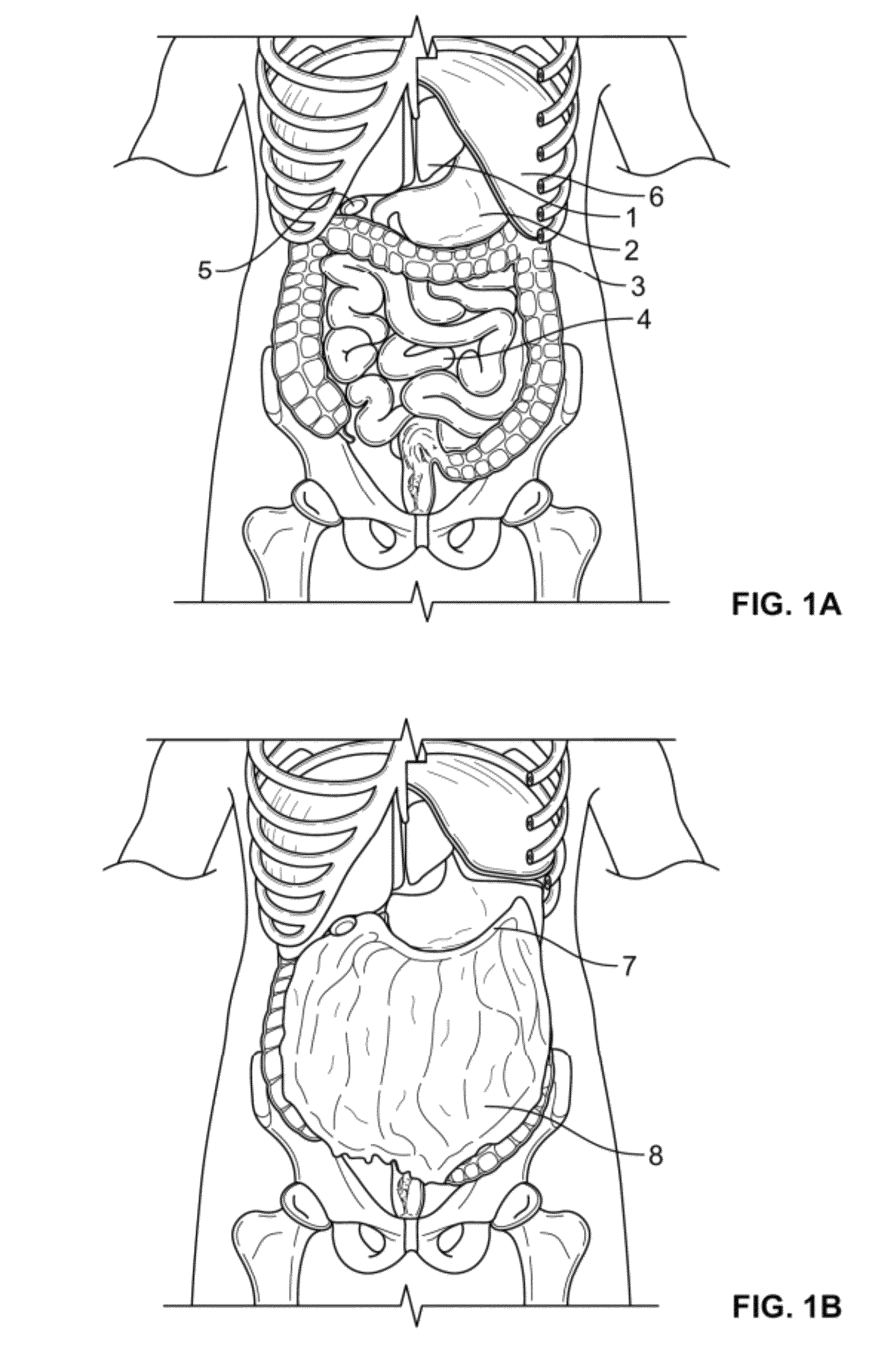
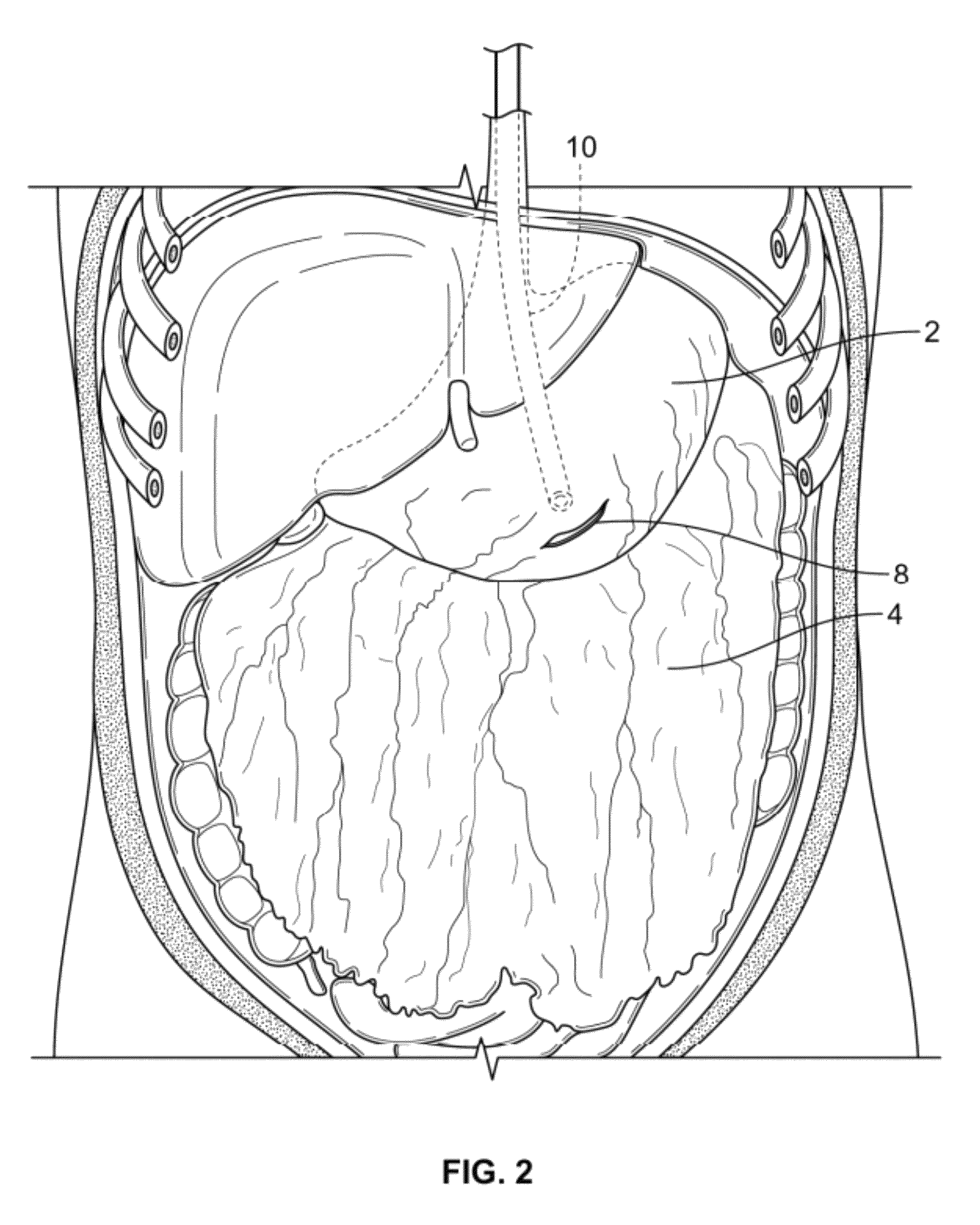
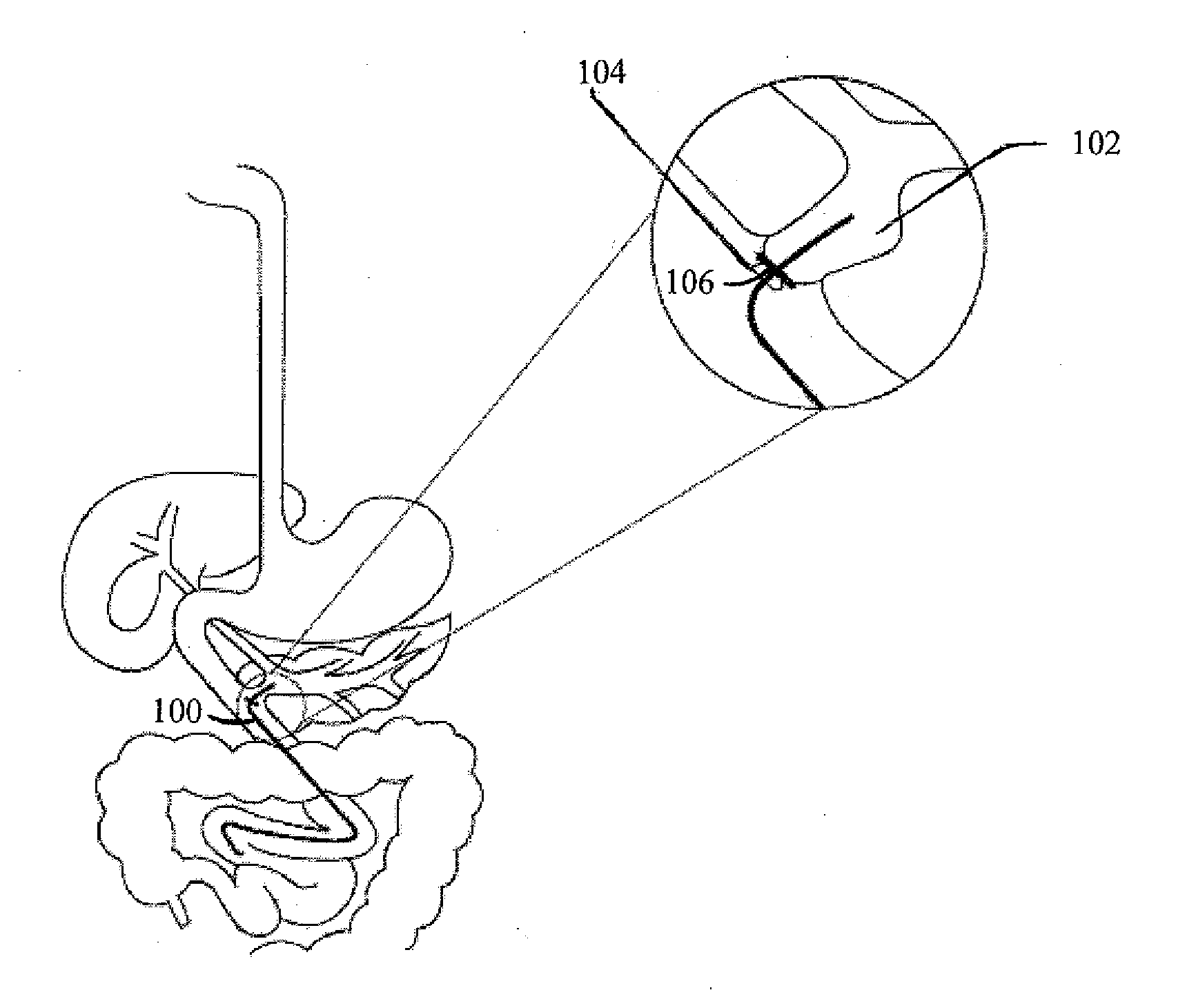
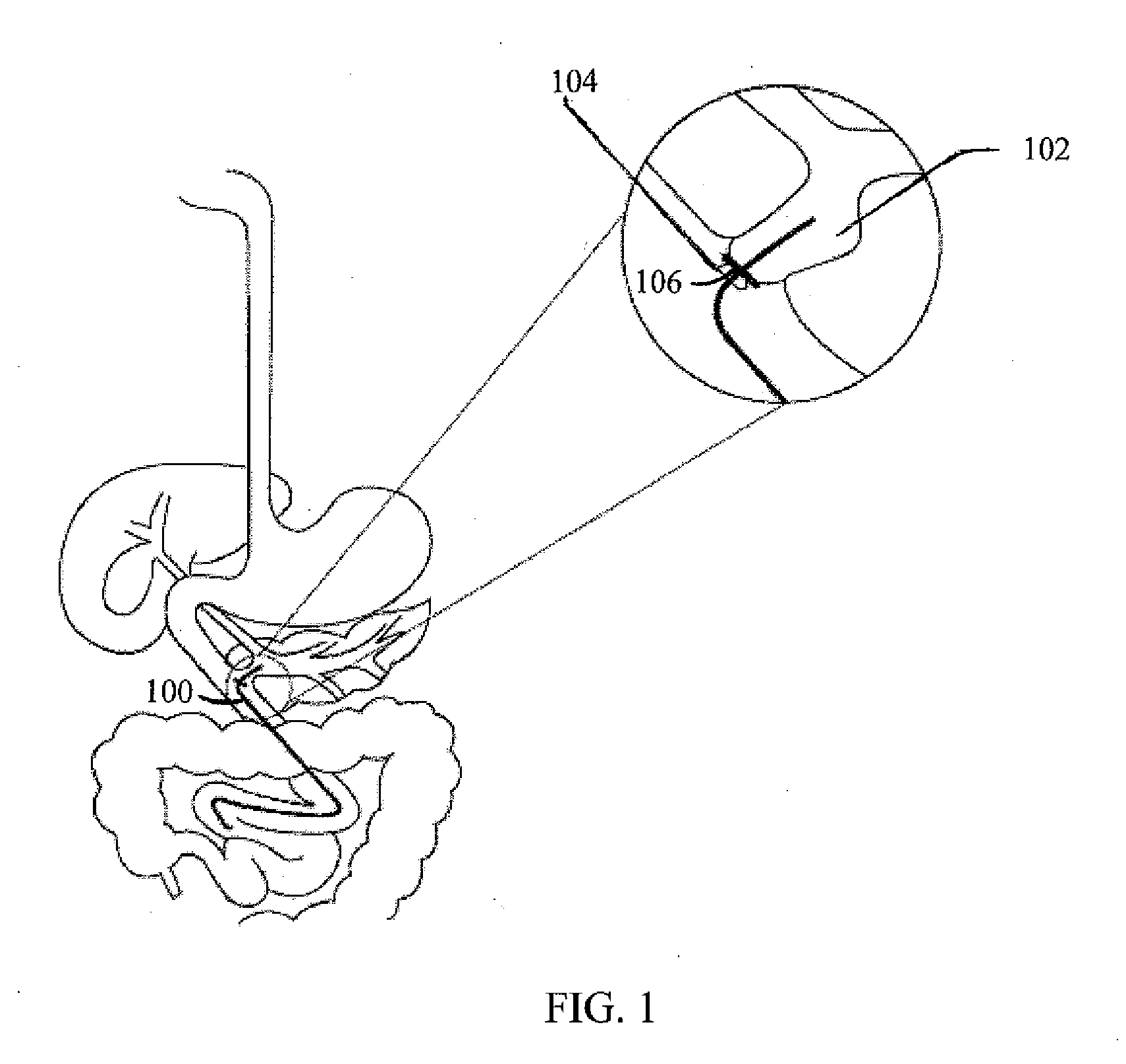
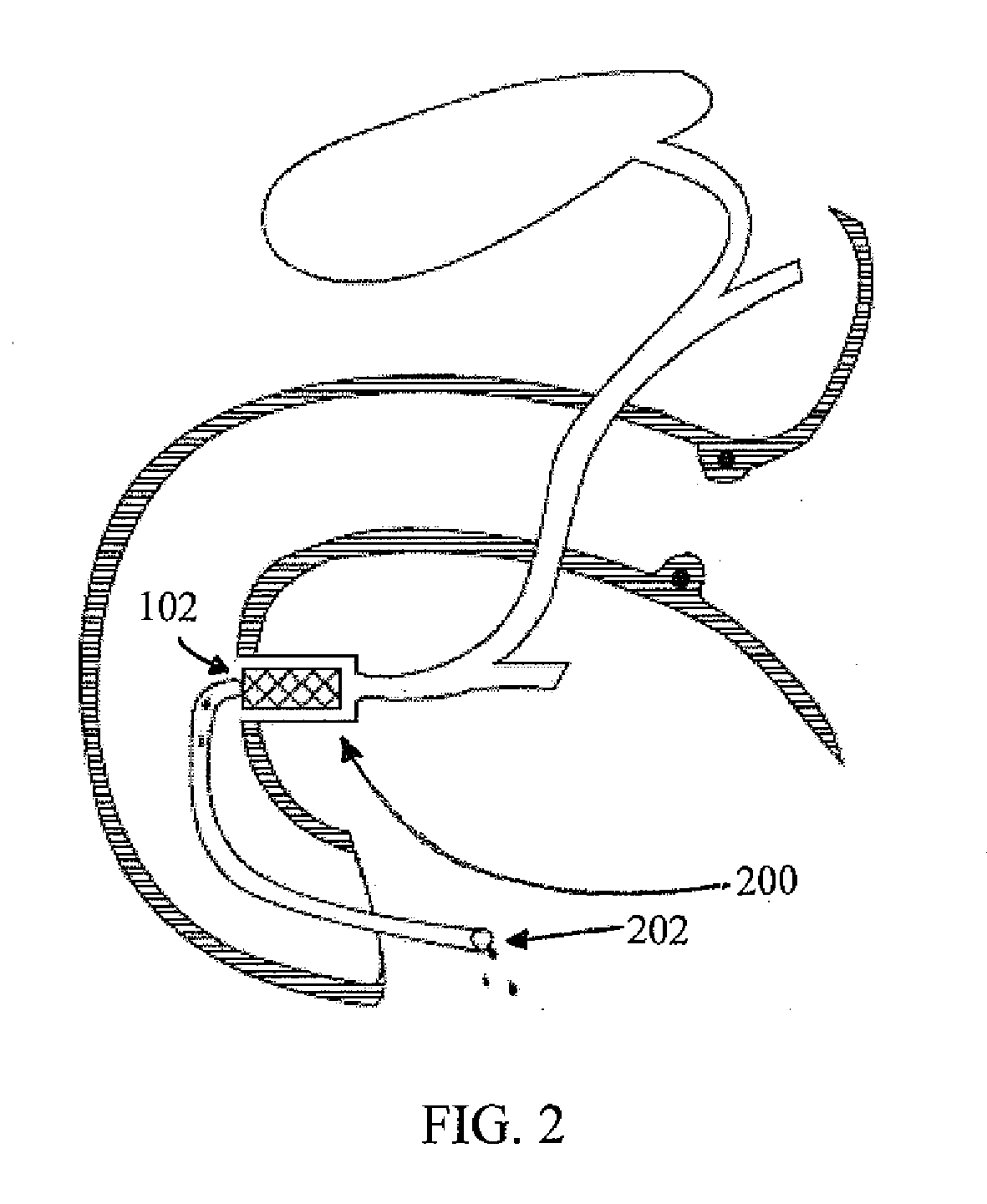
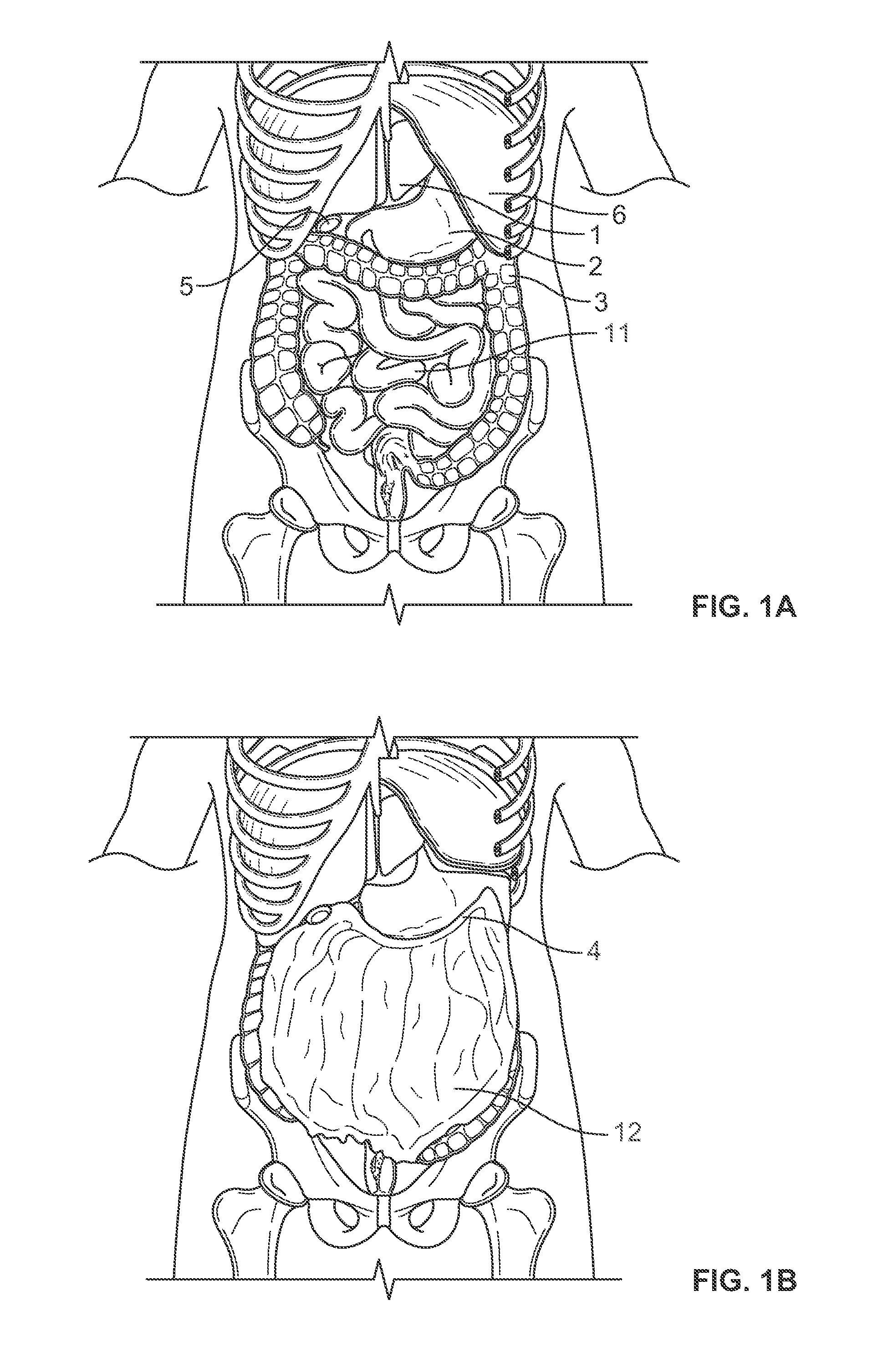
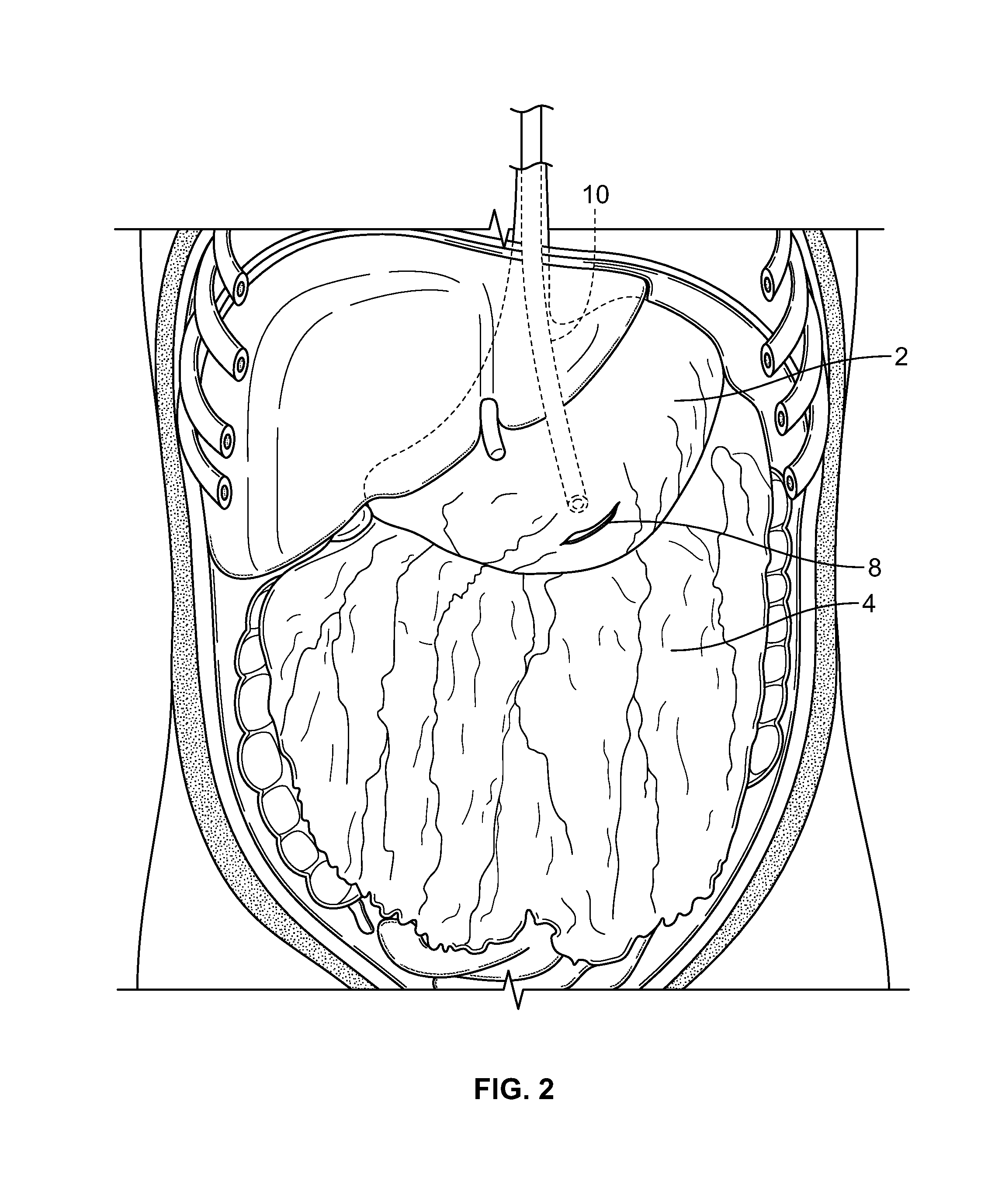
InactiveUS20100210999A1Avoid bleedingReduce morbidityDiagnosticsMedical devicesCo morbidityTissue material
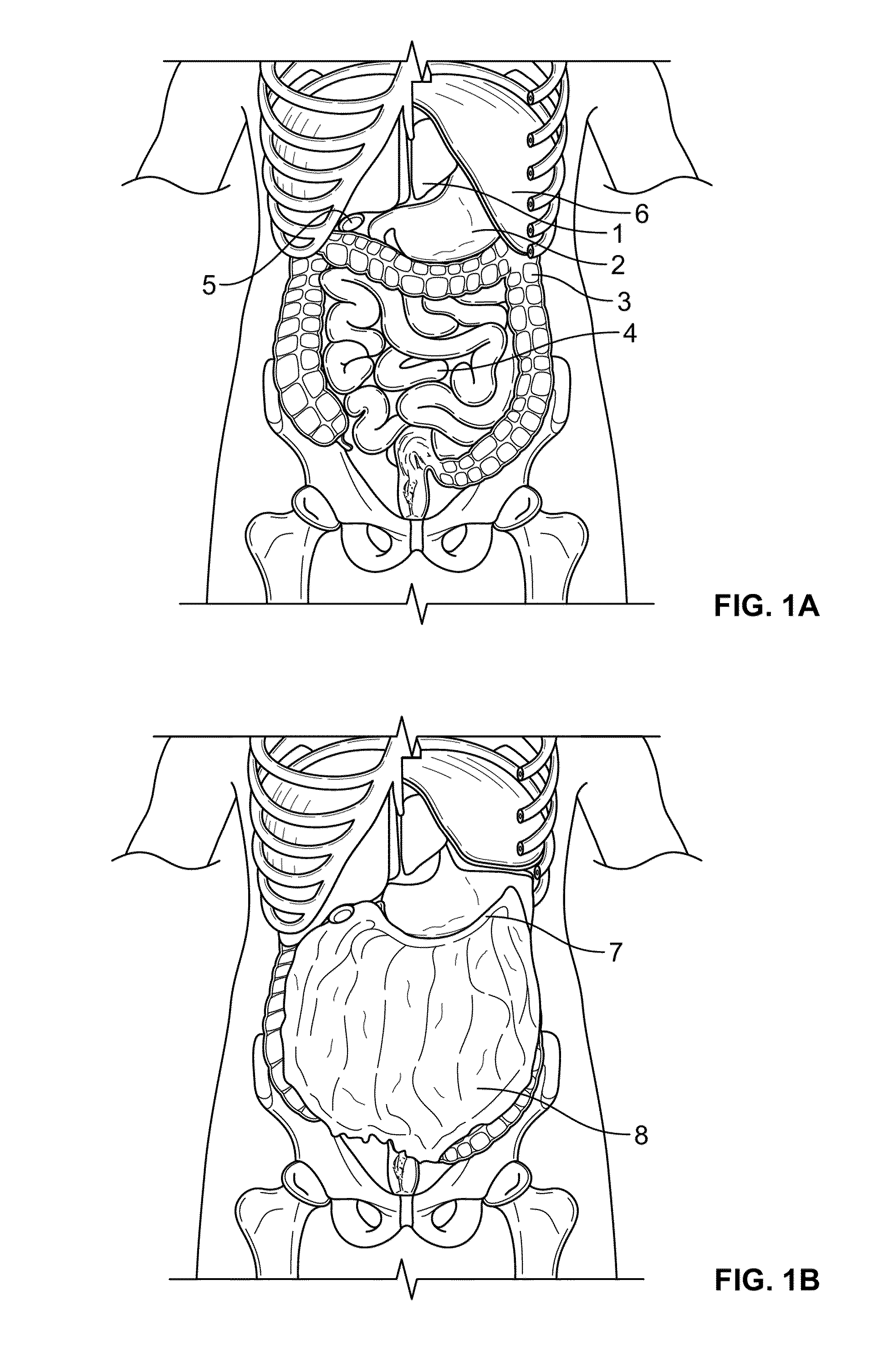
The invention relates to a method of treating obesity, insulin resistance and co-morbidities of these conditions by removing tissue from the abdomen. More specifically, it relates to a method of removing abdominal fat and omentum to which the fat is attached, in order to improve health. The invention includes a device for safely removing this tissue material
Owner:LENR
Coordinated health and human services delivery system and process
A system (200) and method (500) is provided for a coordinated health care service delivery program. The method can include providing services to clients at high risk for chronic disease including co-morbidities and consequent disabilities associated with the chronic disease, linking community and vocational services (130) for facilitating community inclusion to supplement fundamental clinical and economic goals, creating a comprehensive and dynamic individual development plan (222) to involve the client and family members as active program team members for stressing client-centric collaborative goal setting, and applying action learning (226) to promote behavior modification and lifestyle change.
Owner:THE TRINITY MANAGEMENT GROUP
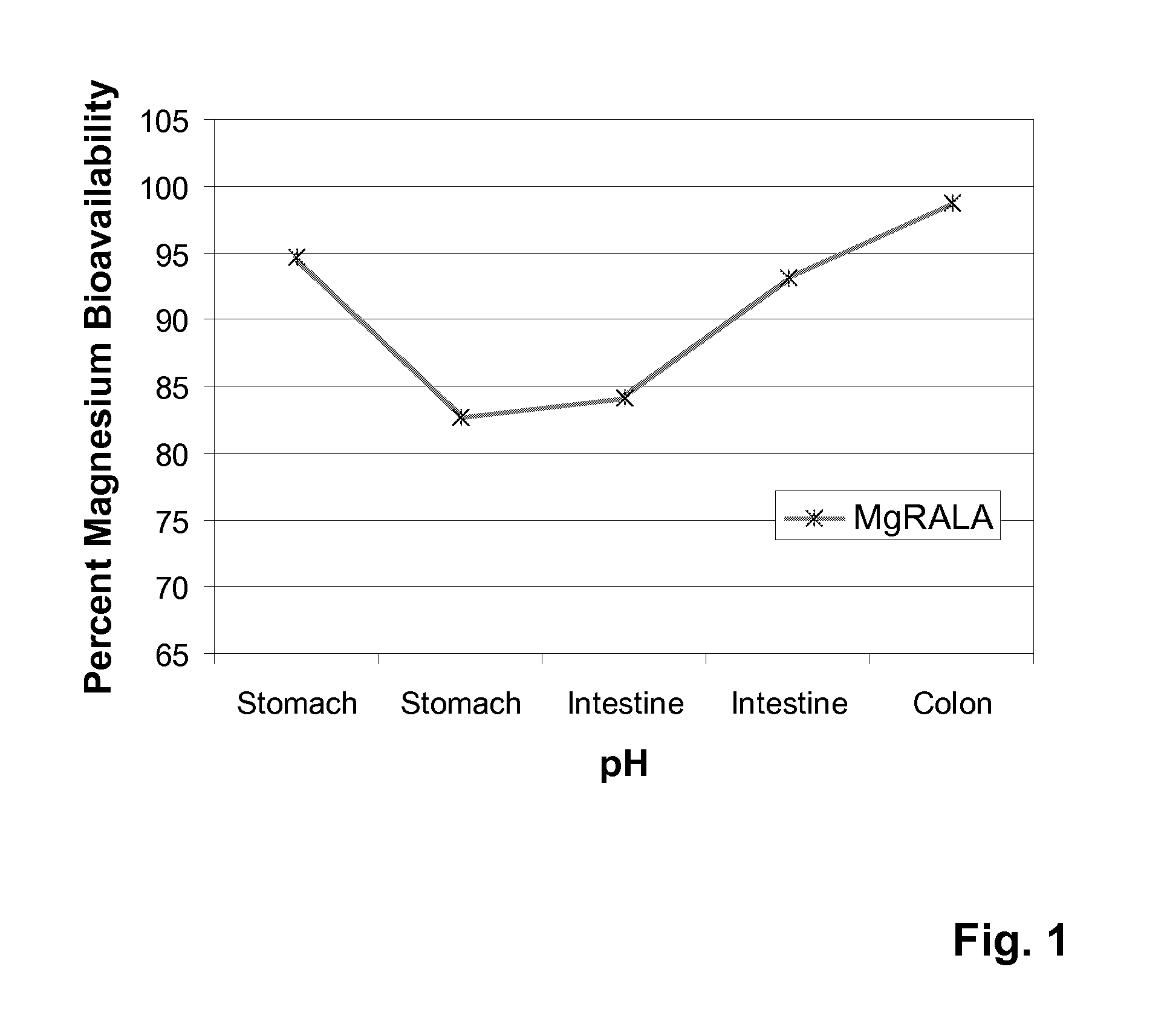
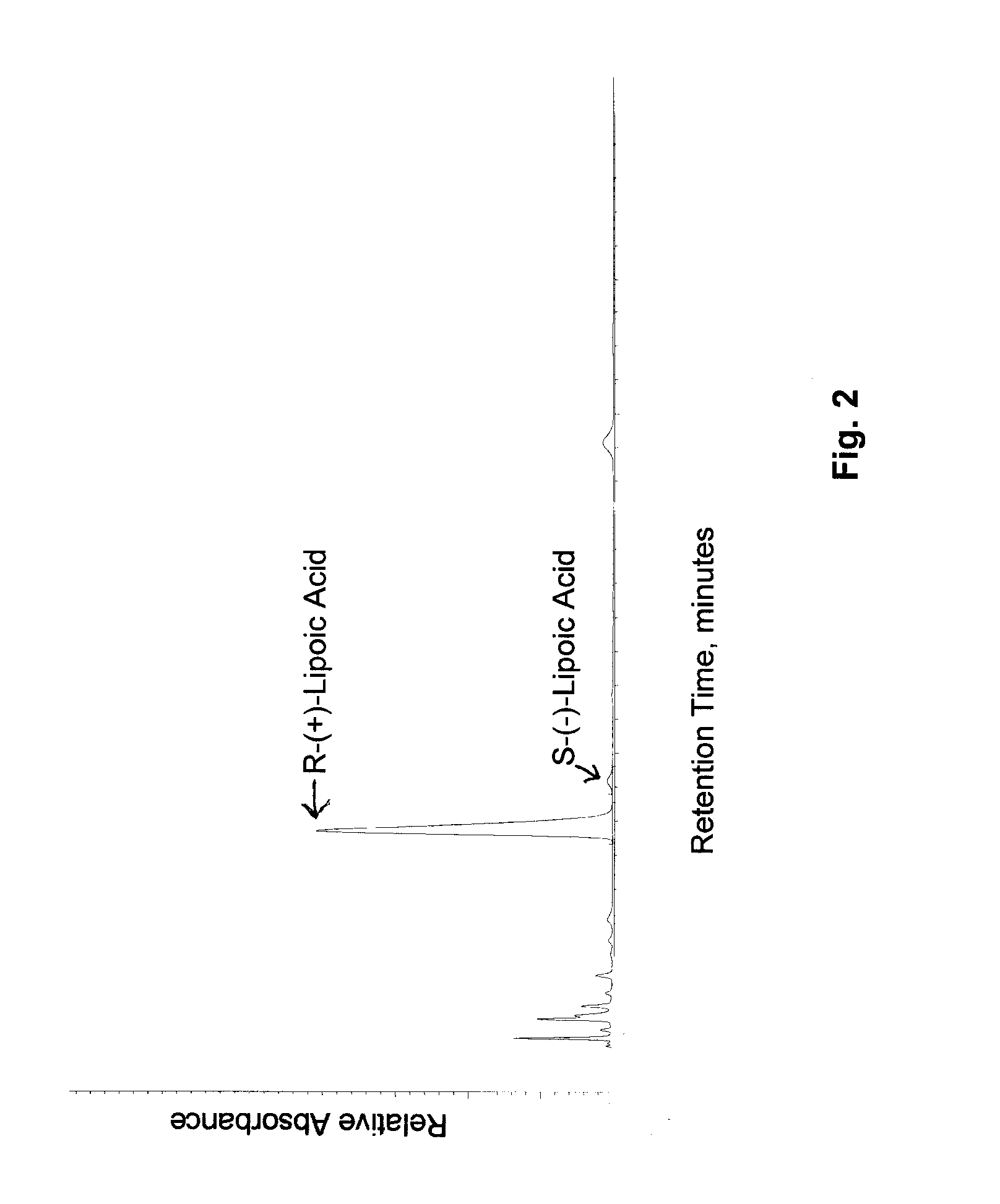
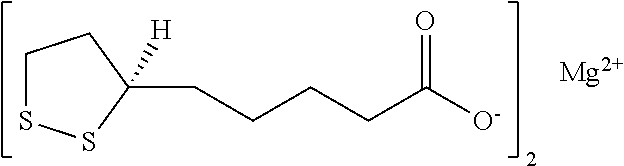
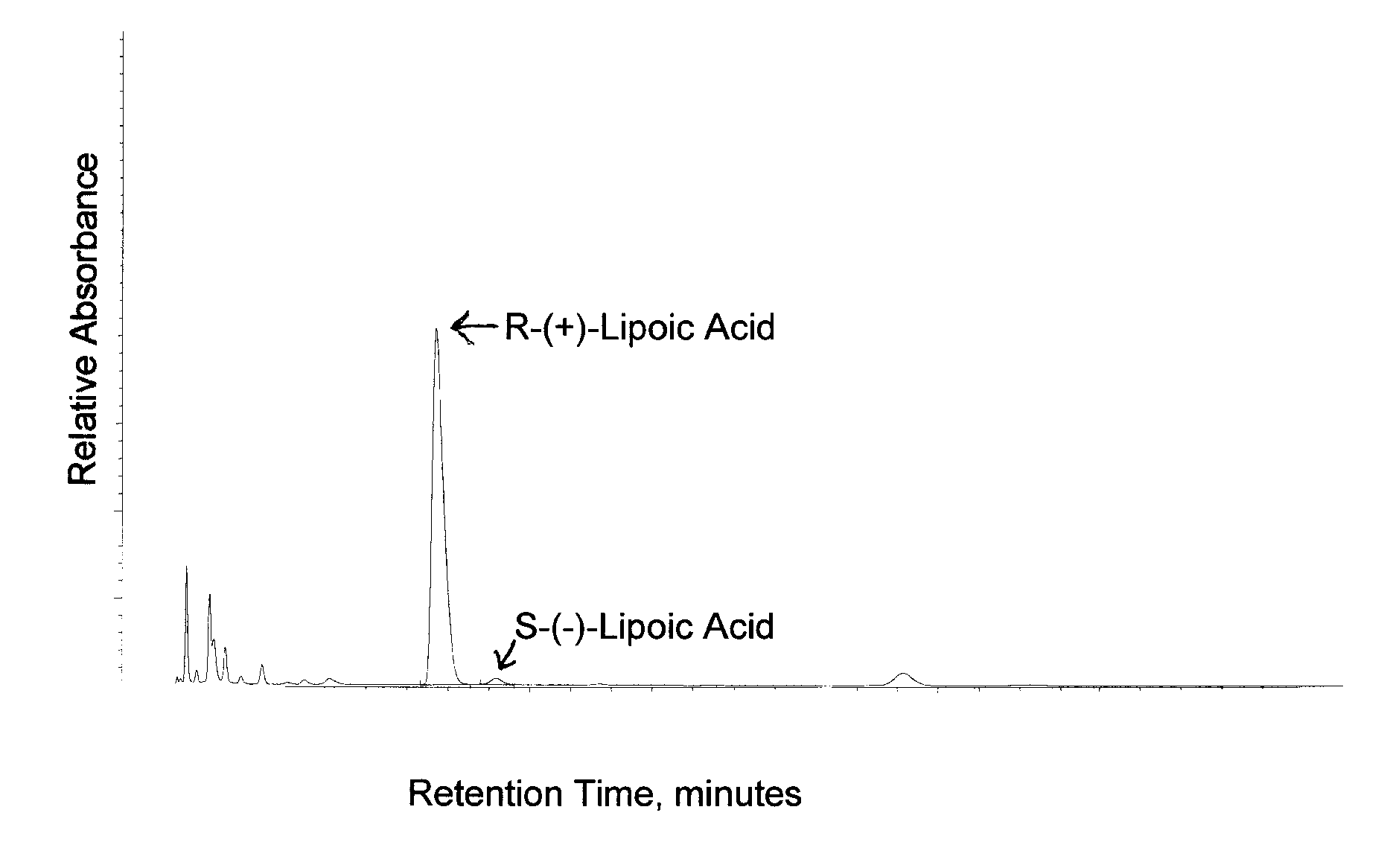
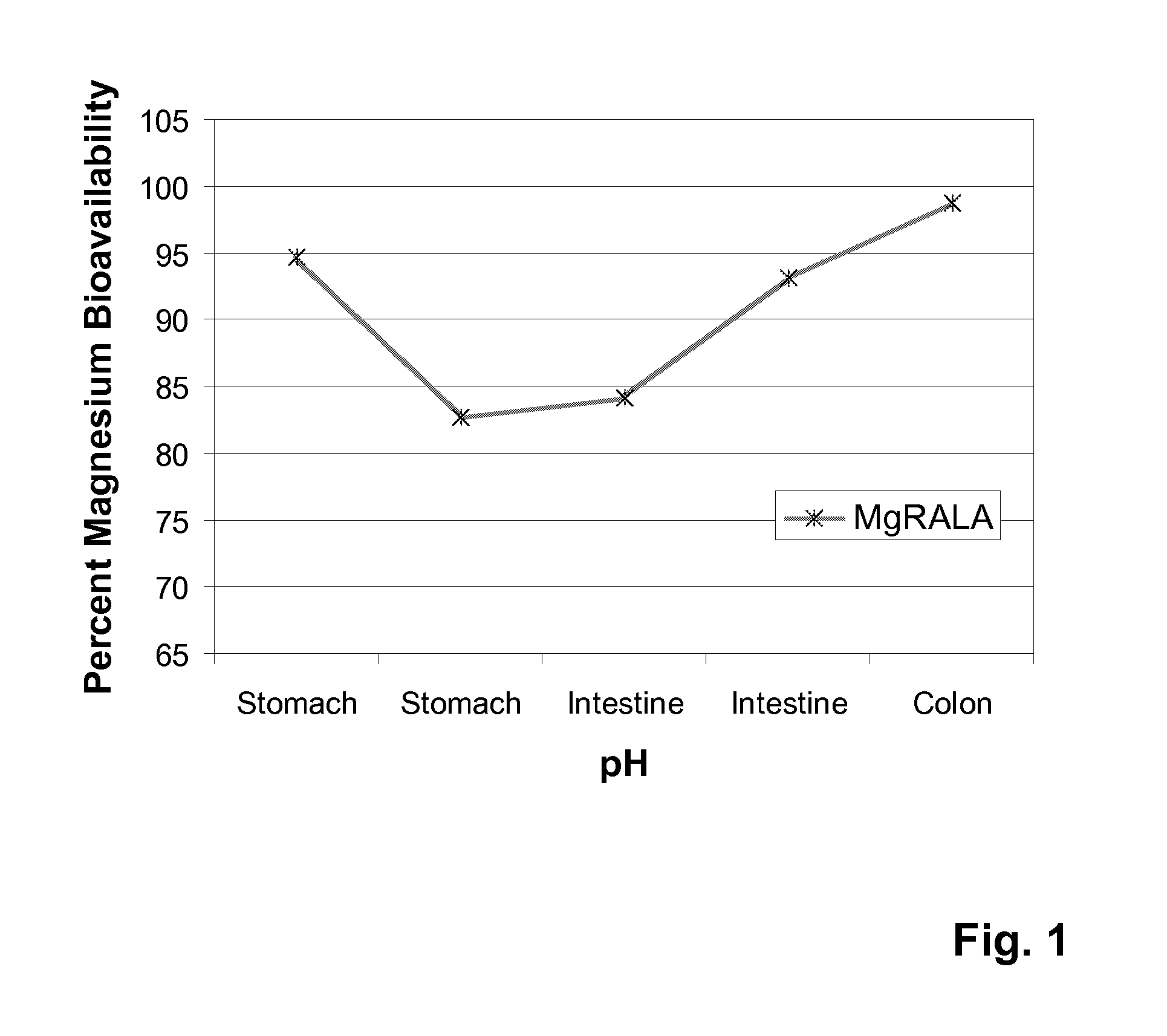
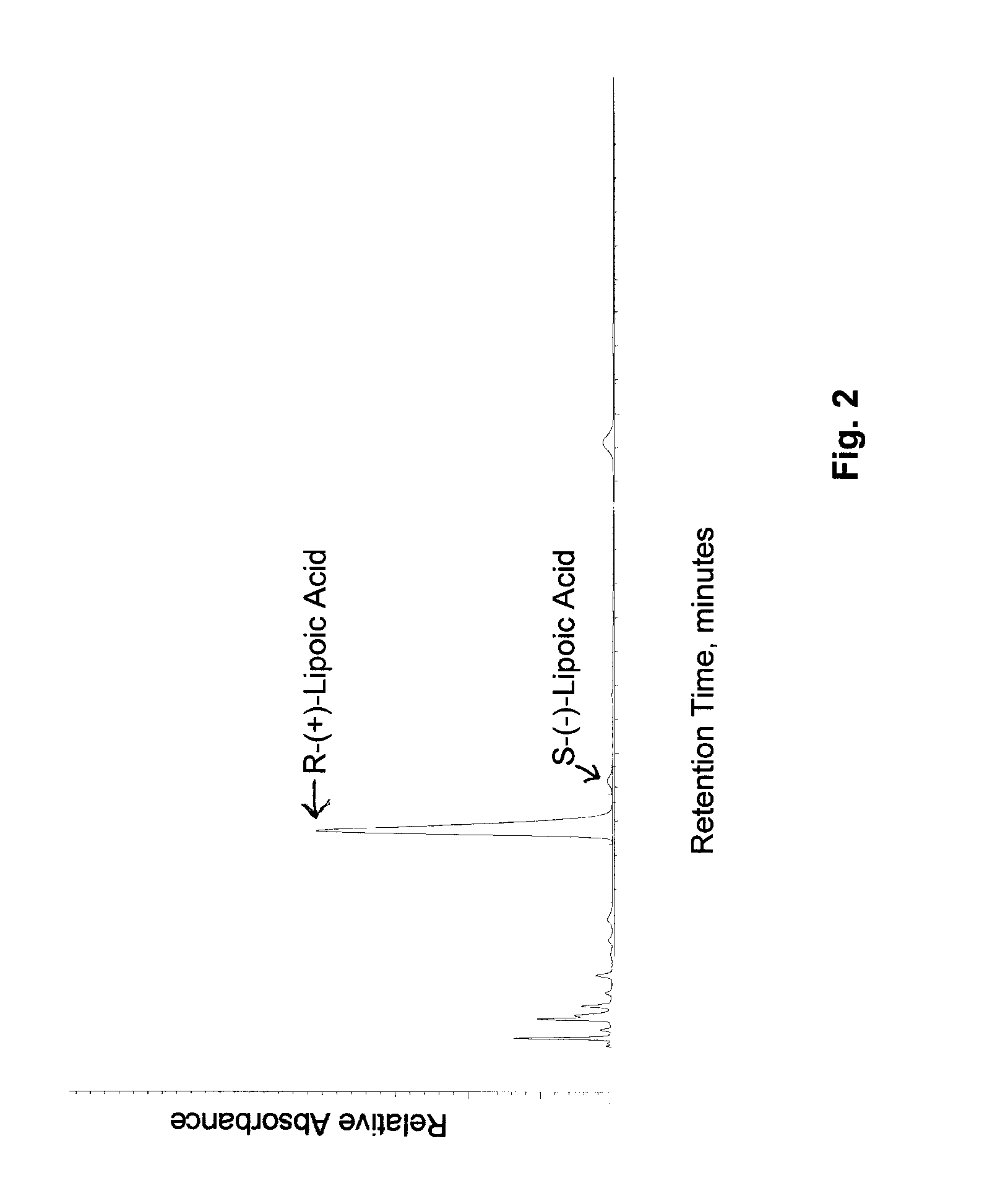
Stable, water-insoluble r-(+)-alpha-lipoic acid salt useful for the treatment of diabetes mellitus and its co-morbidities
The present invention relates to oral nutritional and therapeutic products which are useful for preventing or treating compensated and decompensated insulin resistance and associated diseases and sequelae, or diabetes mellitus and its sequelae, complications, and co-morbidities, comprising magnesium R-(+)-alpha-lipoate.
Owner:BIOLINK LIFE SCI
Specific markers for metabolic syndrome
Owner:F HOFFMANN LA ROCHE & CO AG
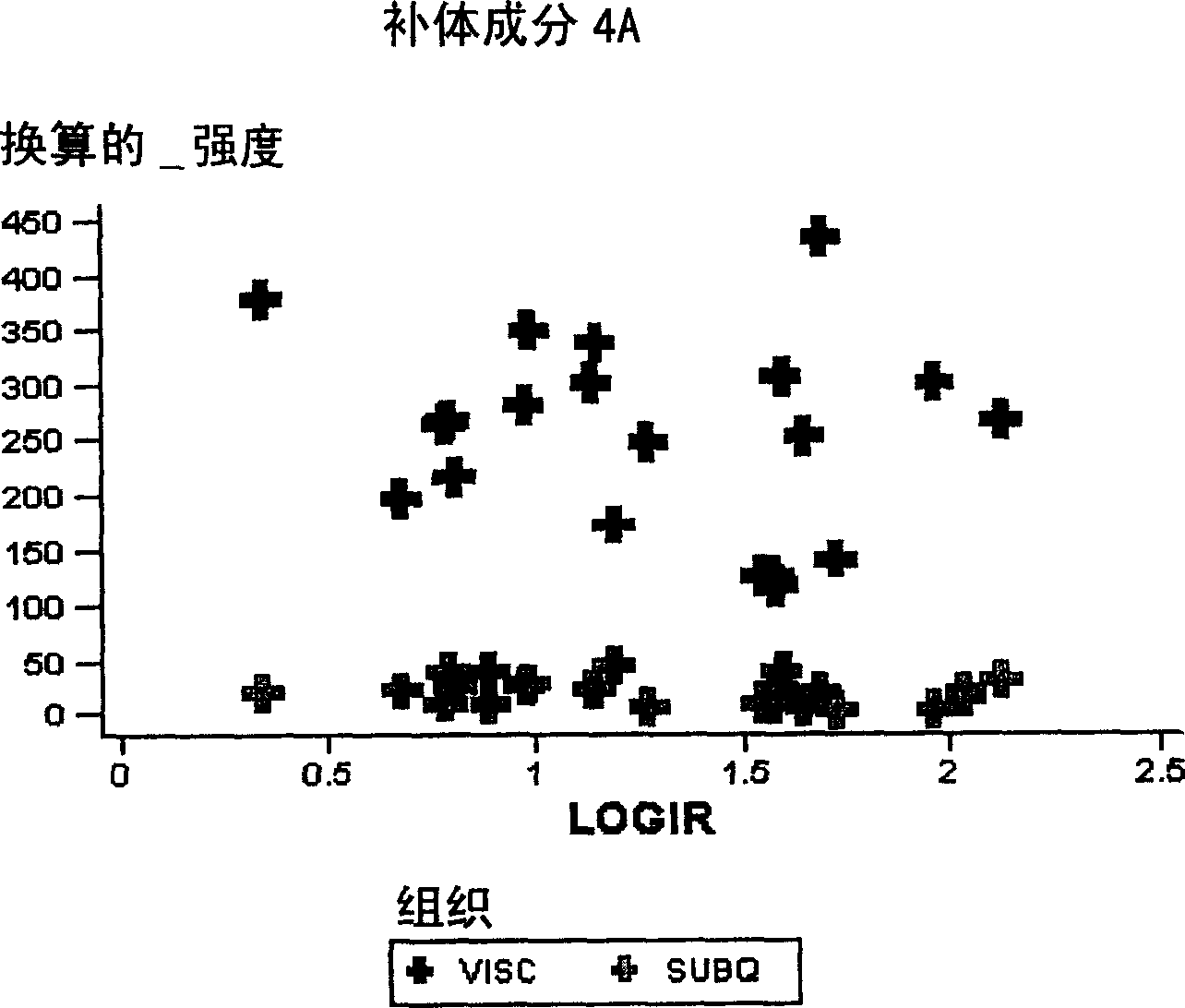
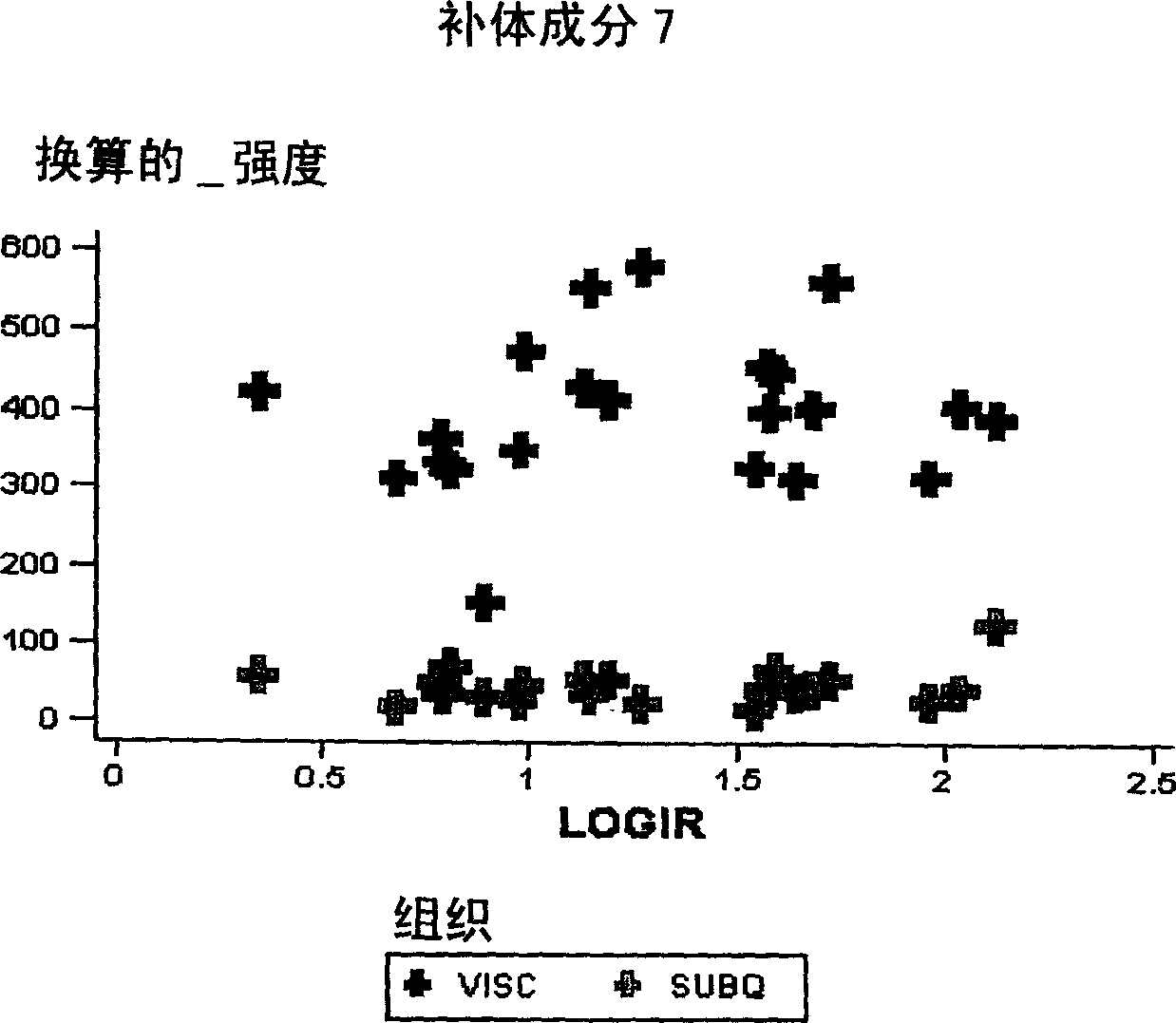
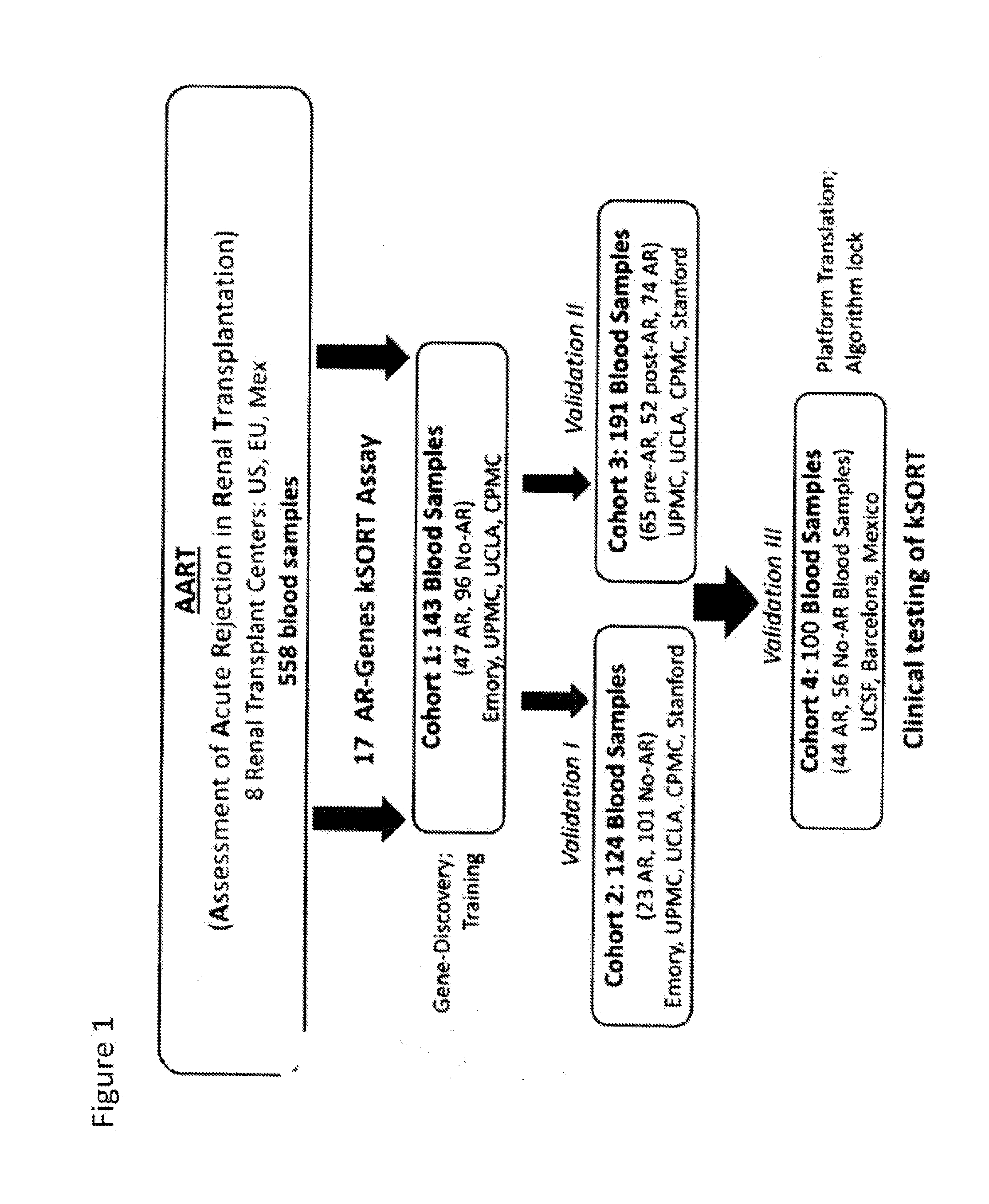
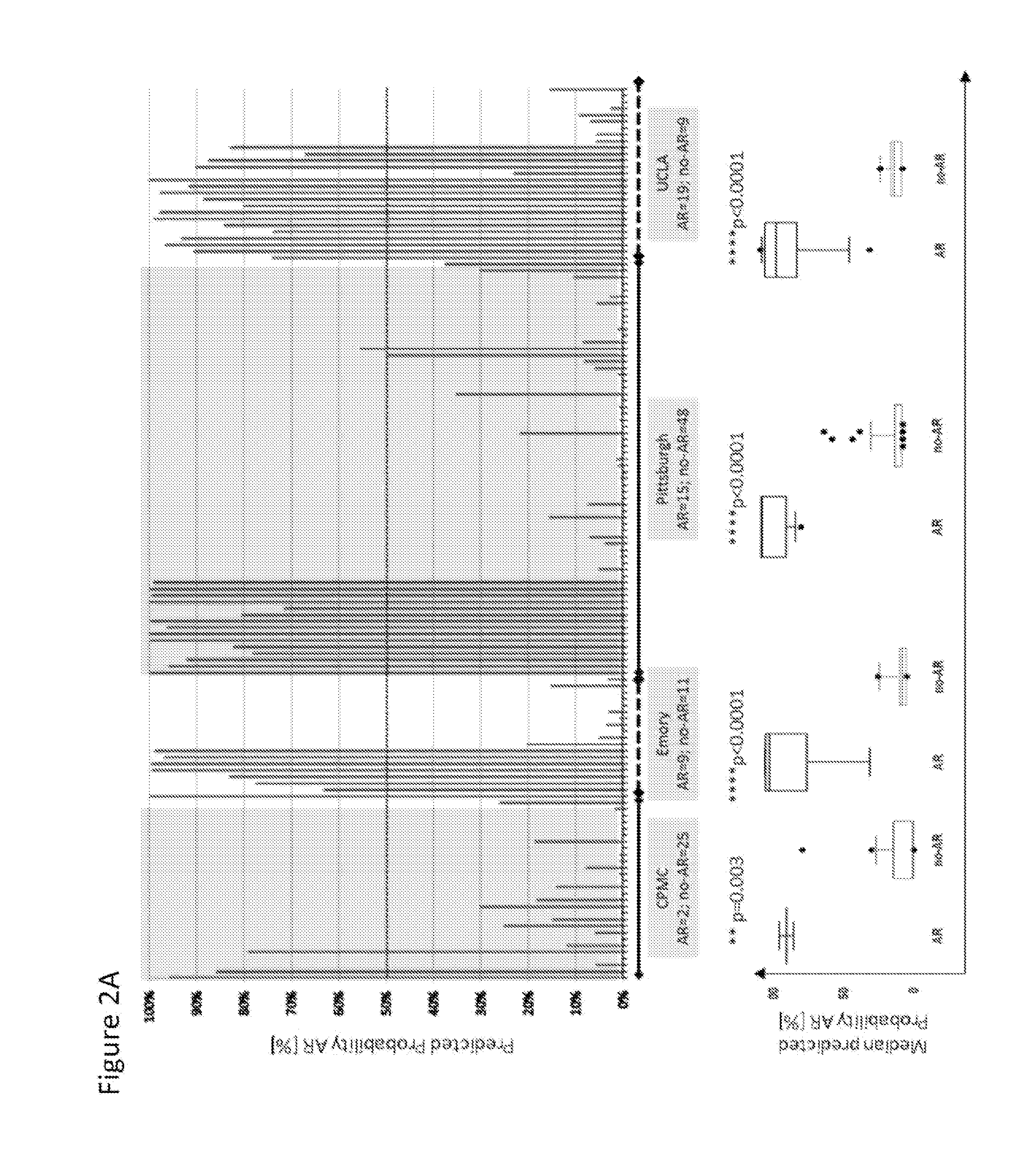
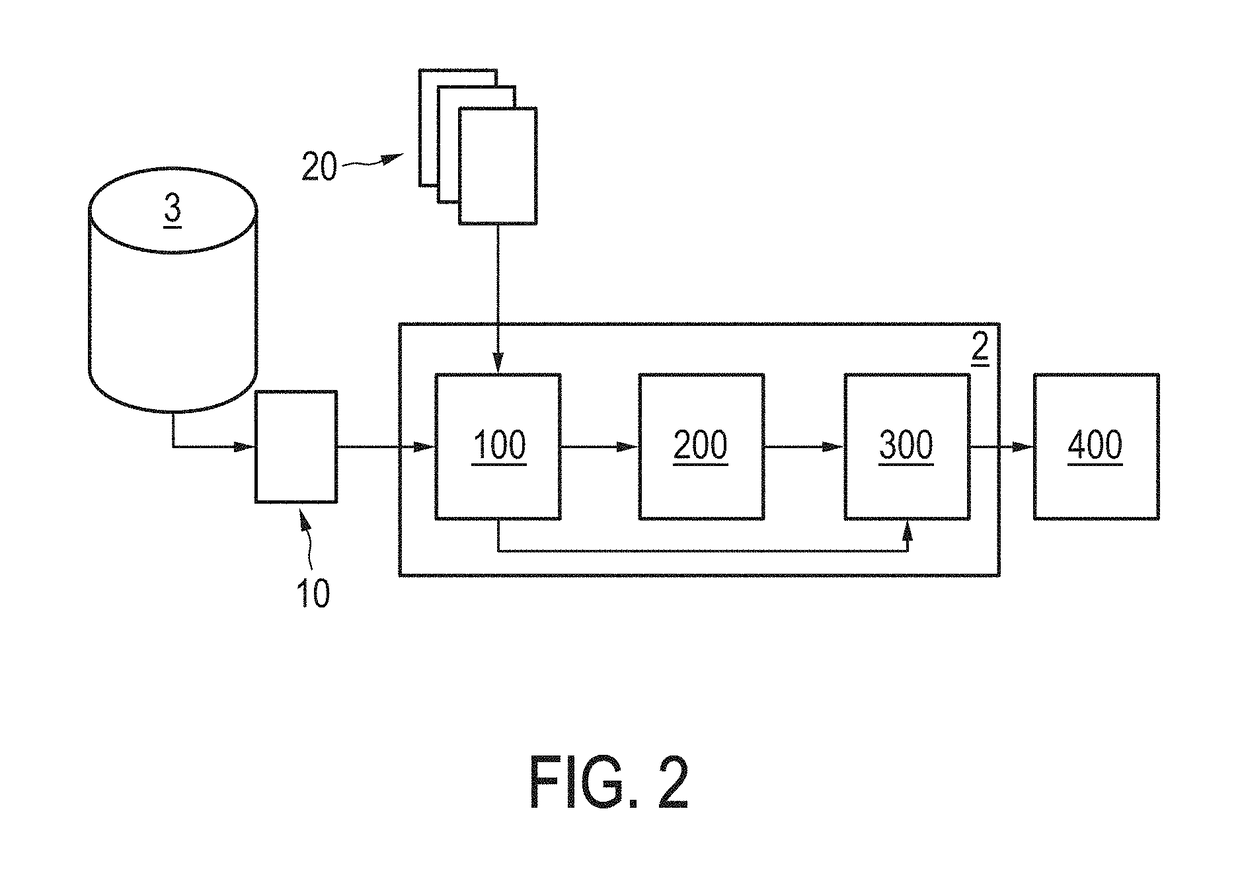
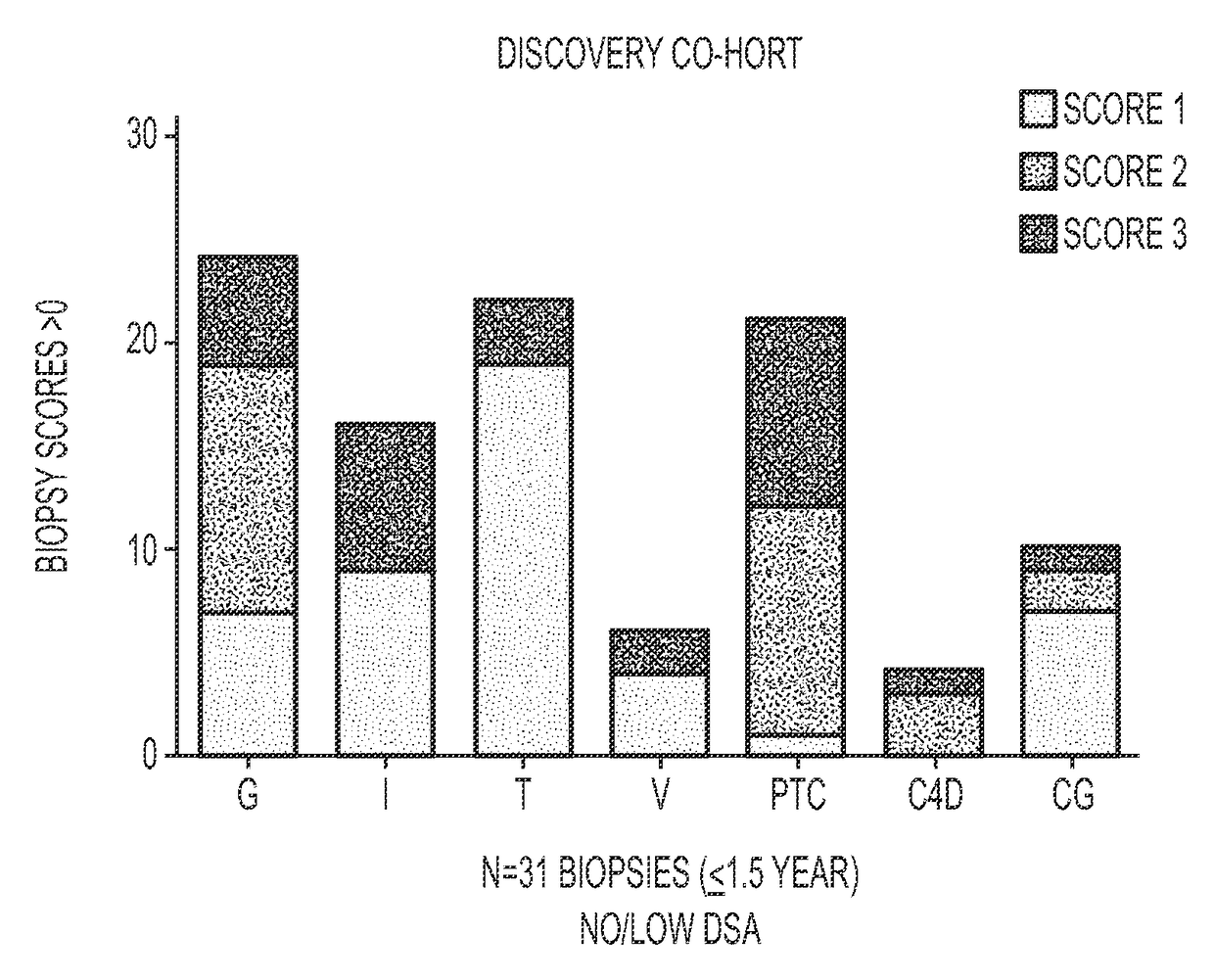
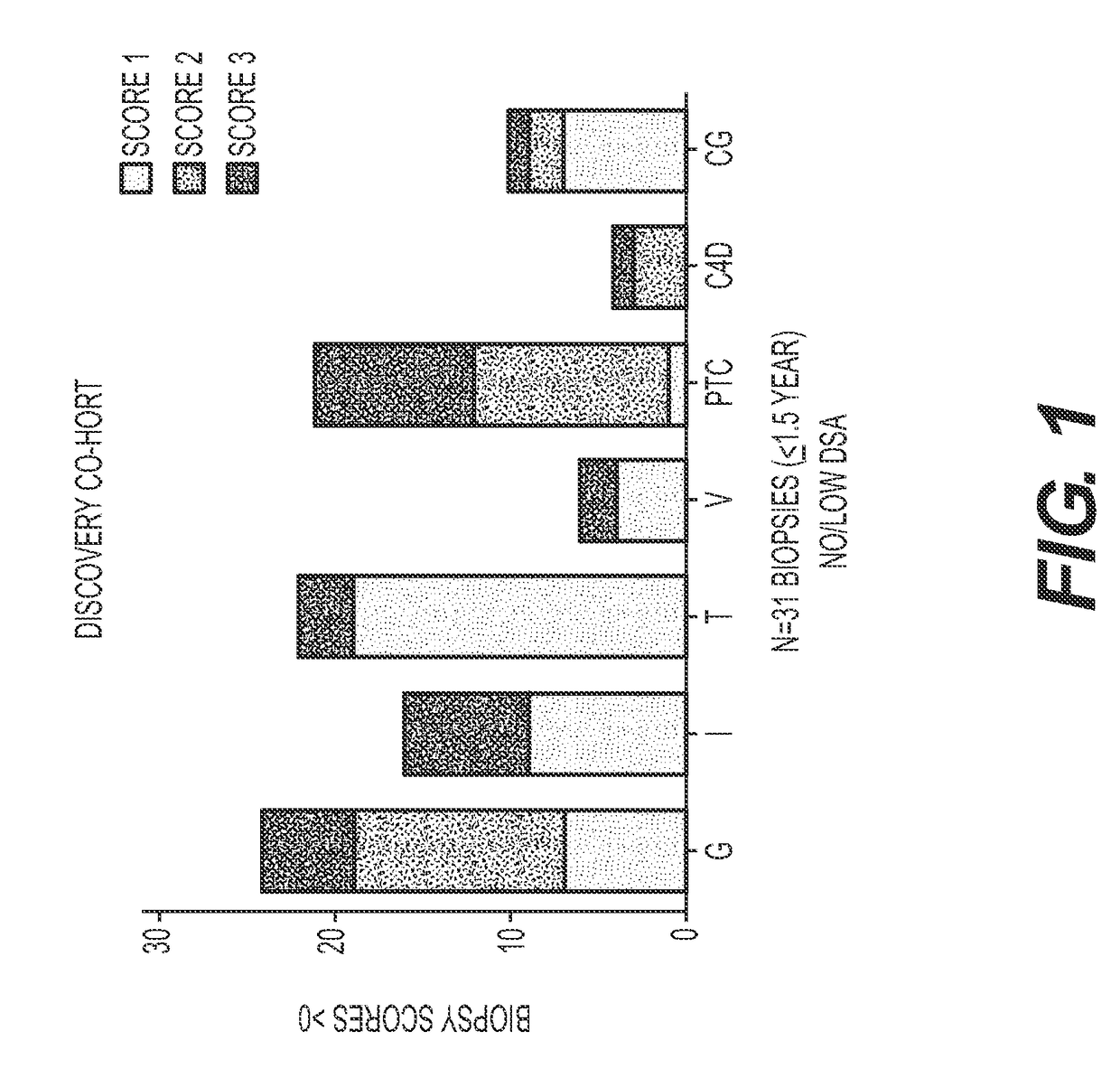
Compositions and methods for assessing acute rejection in renal transplantation
InactiveUS20160348174A1Microbiological testing/measurementUrinary disorderNephrosisEnd stage renal disease
Provided herein are methods, compositions, and kits for diagnosing acute rejection of renal transplants using the gene expression profile of sets of classifier genes. Such methods and compositions are independent of external confounders such as recipient age, transplant center, RNA source, assay, cause of end-stage renal disease, co-morbidities, immunosuppression usage, and the like.
Owner:IMMUCOR GTI DIAGNOSTICS
Methods and devices for removing omental tissue
InactiveUS8206386B2Reduce morbidityEliminates collateral effectDiagnosticsMedical devicesCo morbidityTissue material
The invention relates to a method of treating obesity, insulin resistance and co-morbidities of these conditions by removing tissue from the abdomen. More specifically, it relates to a method of removing abdominal fat and omentum to which the fat is attached, in order to improve health. The invention includes a device for safely removing this tissue material.
Owner:LENR
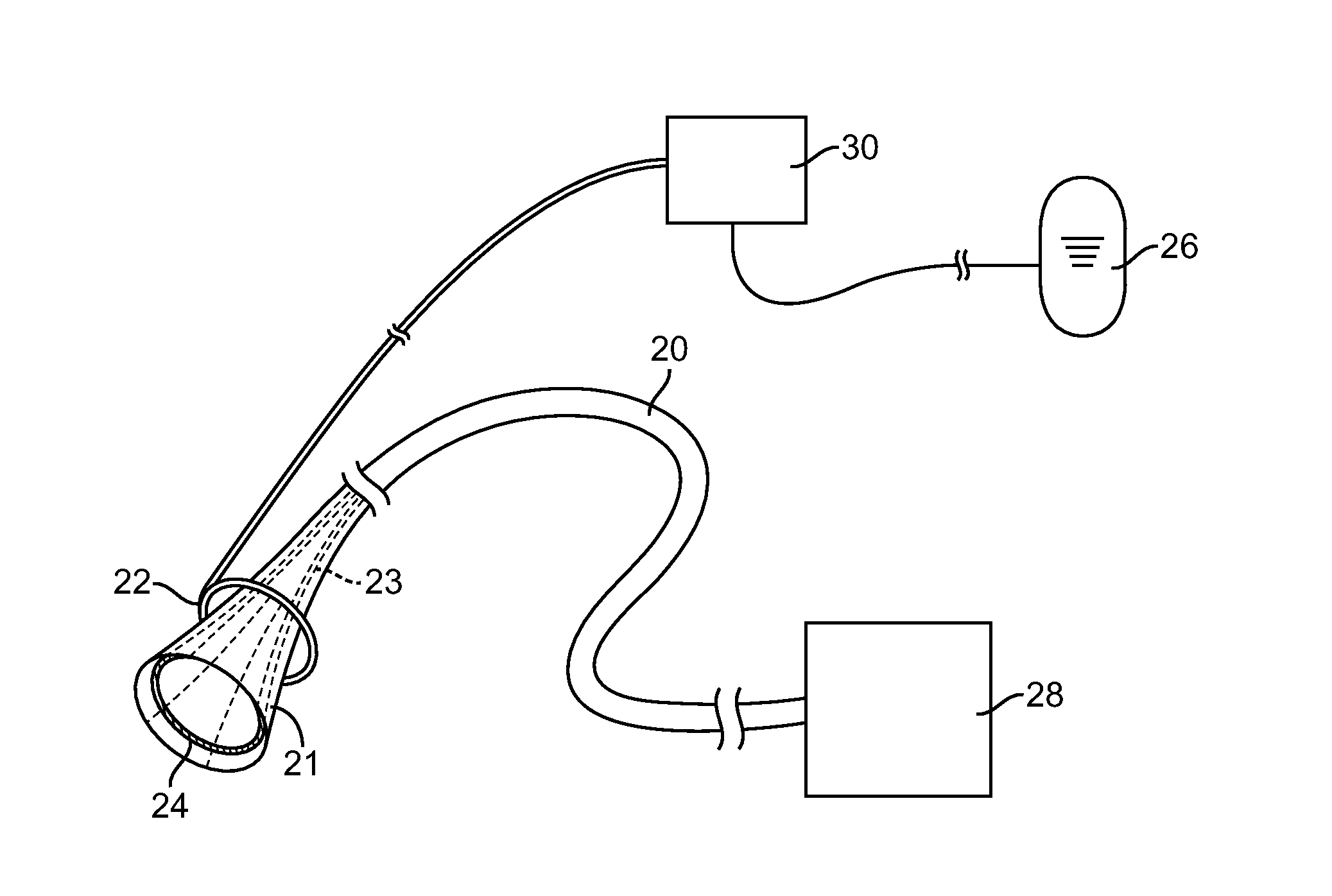
Method For Surgically Treating A Patient By Deactivating A Portion Of The Digestive Enzymes
InactiveUS20110106225A1Speed deliveryExcessive weight lossWound drainsMedical devicesCo morbidityNon invasive
The present invention generally provides for correcting an imbalance between caloric intake and caloric expenditure in patients, as well as for treating co-morbidities often associated therewith, which is non-invasive or minimally invasive and which may be reversible. More specifically, the present invention provides systems which cause metabolic improvement in a patient by controlling the amount of bile available for food breakdown or by controlling the effective absorption time and area by delivering bile to selected locations in the intestinal tract. These methods and devices fall under three general categories: bile diversion systems, bile manipulation systems, and surgical methods.
Owner:ETHICON ENDO SURGERY INC
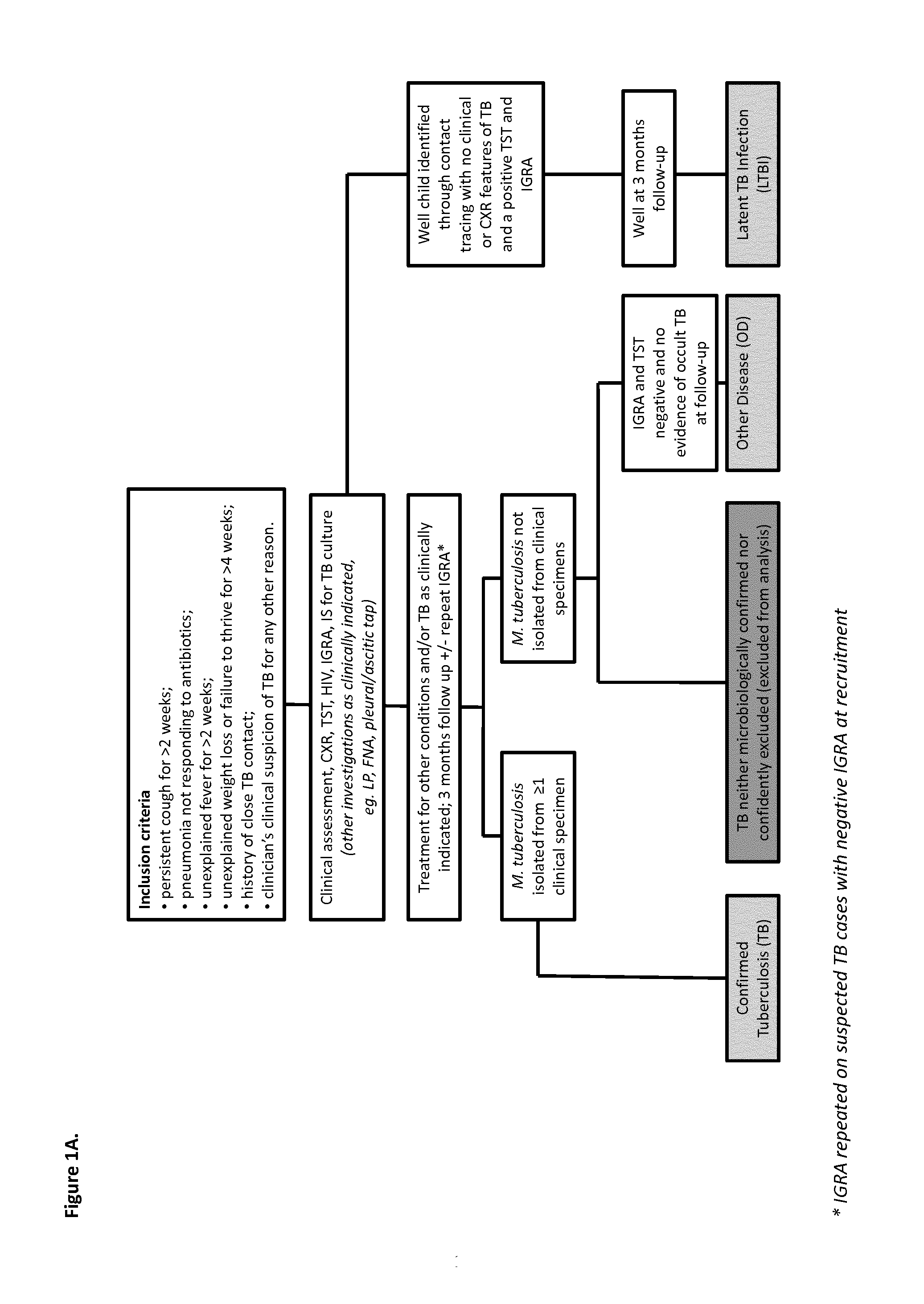
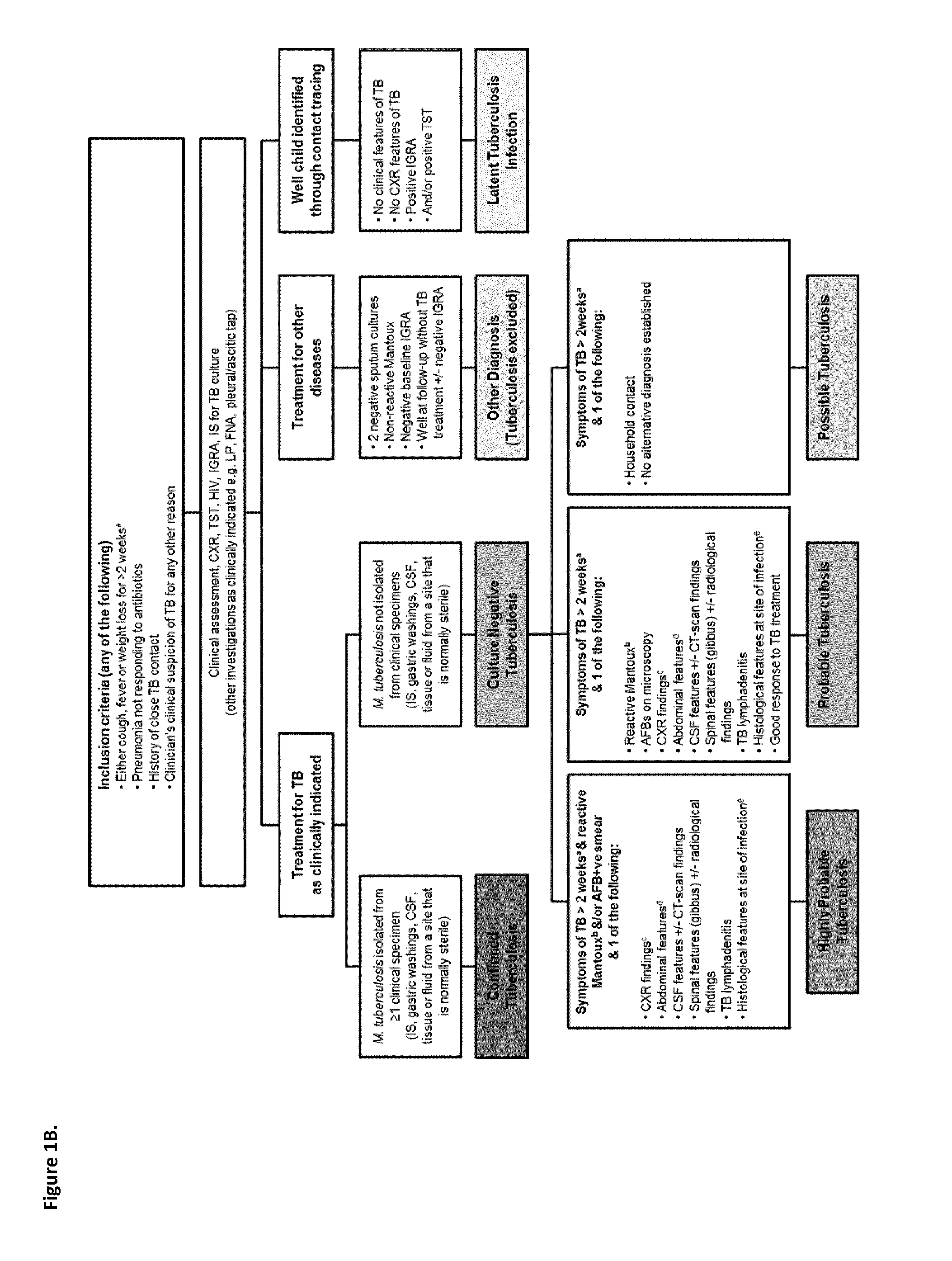
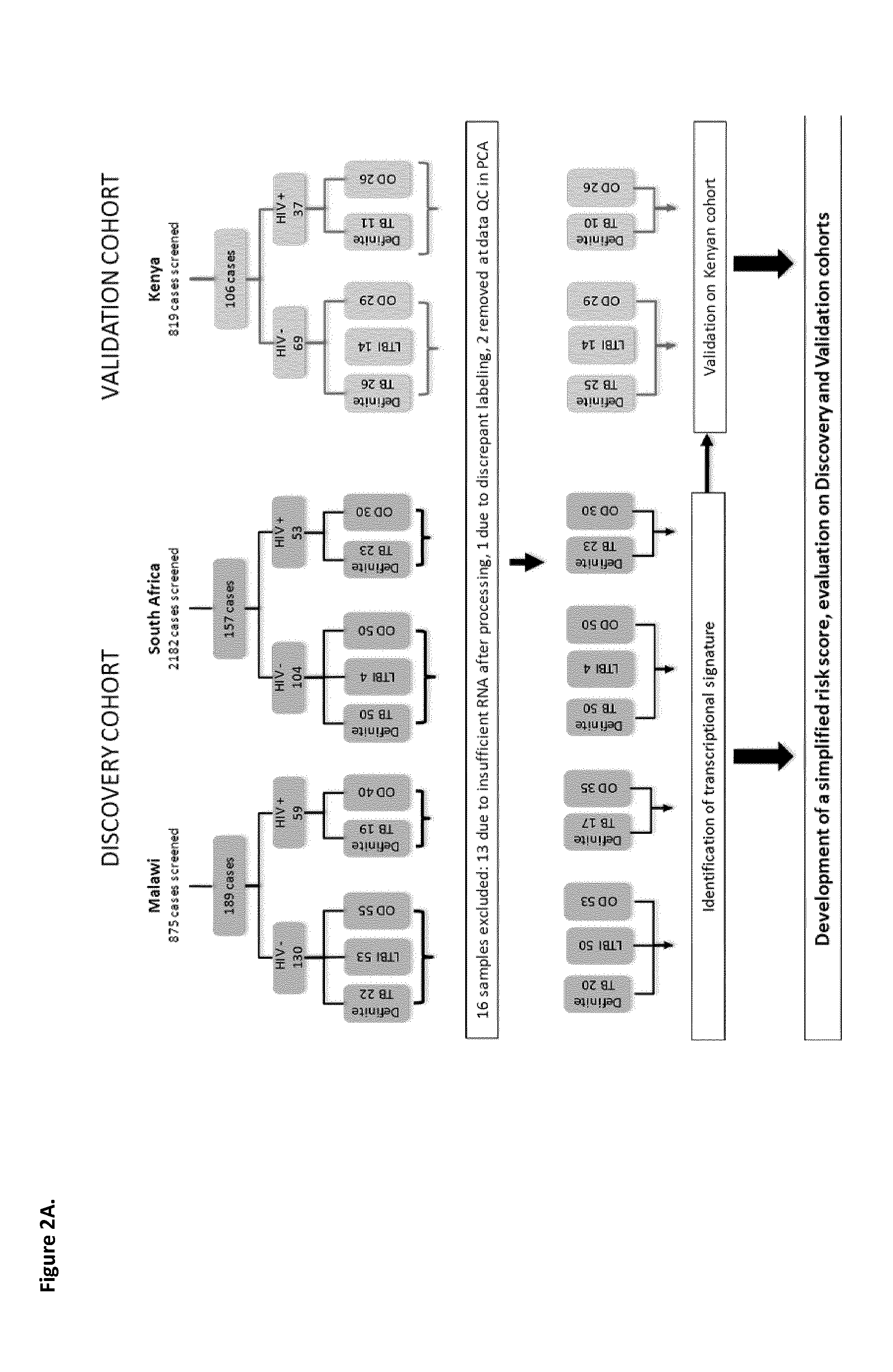
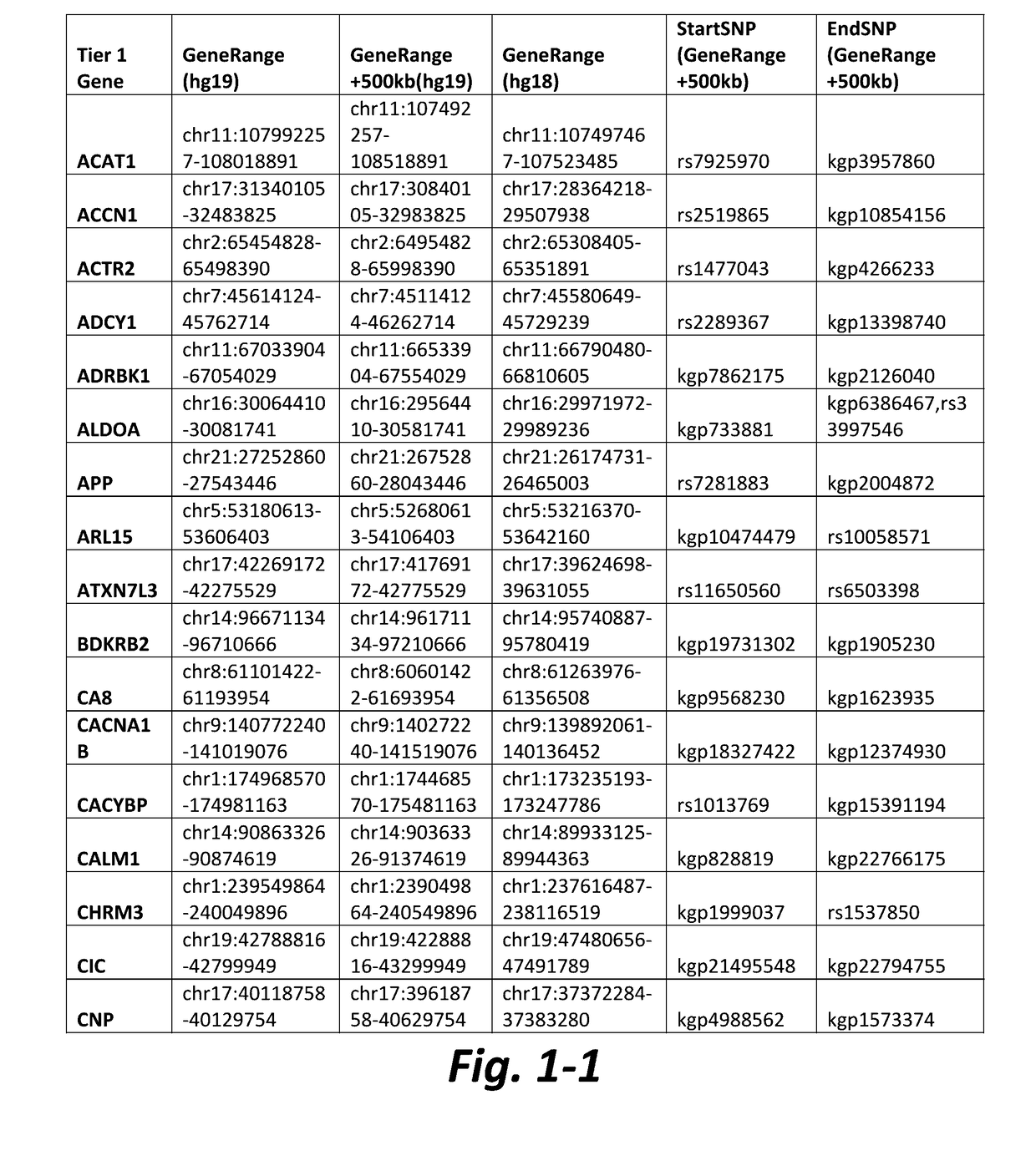
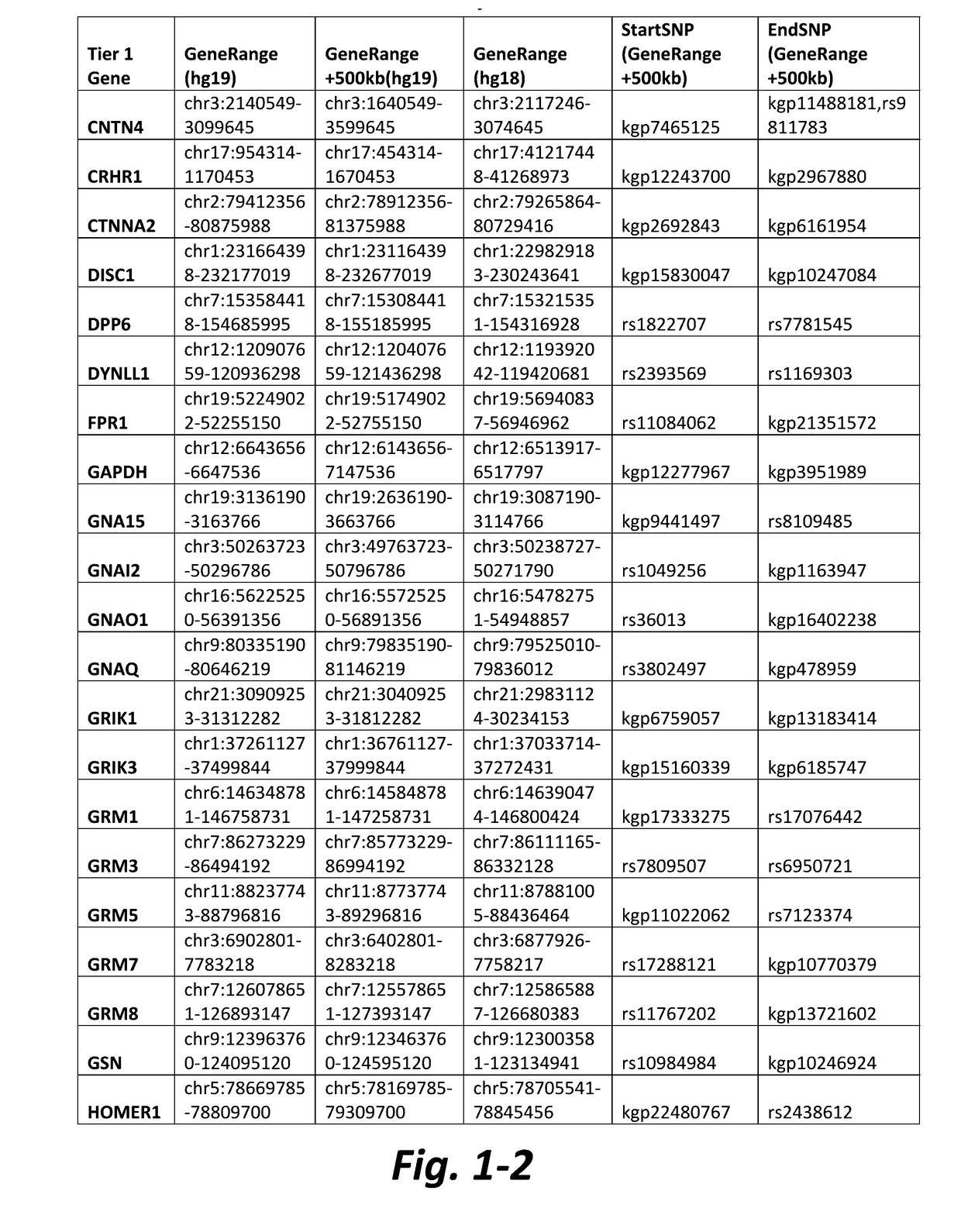
Method of detecting active tuberculosis in children in the presence of a co-morbidity
InactiveUS20150284780A1Robust and accurate identificationAppropriate treatmentMicrobiological testing/measurementLibrary screeningDisease riskCo morbidity
The present disclosure relates to a method of distinguishing active paediatric TB in the presence of a complicating factor, for example, latent TB and / or co-morbidities, such as those that present similar symptoms to TB in a child. The method employs a 42 gene signature and / or a 51 gene signature. The disclosure also relates to a gene signature employed in the method, a bespoke gene chip for use in the method and a disease risk score obtainable from the method.
Owner:IMPERIAL INNOVATIONS LTD
Nonselective metabotropic glutamate receptor activators for treatment of attention deficit disorder and 22q syndrome
ActiveUS20170105985A1Reduce severityRelieve symptomsOrganic active ingredientsNervous disorderCo morbidityAttention deficits
This application relates to methods of treating attention deficit hyperactivity disorder (ADHD), 22q deletion and / or duplication syndrome, and co-morbidities with a nonselective activator of metabotropic glutamate receptors, such as fasoracetam, for example, in subjects having a genetic alteration in at least one metabotropic glutamate receptor (mGluR) network gene.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Multimarker panel based on PIGF for diabetes types 1 and 2
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
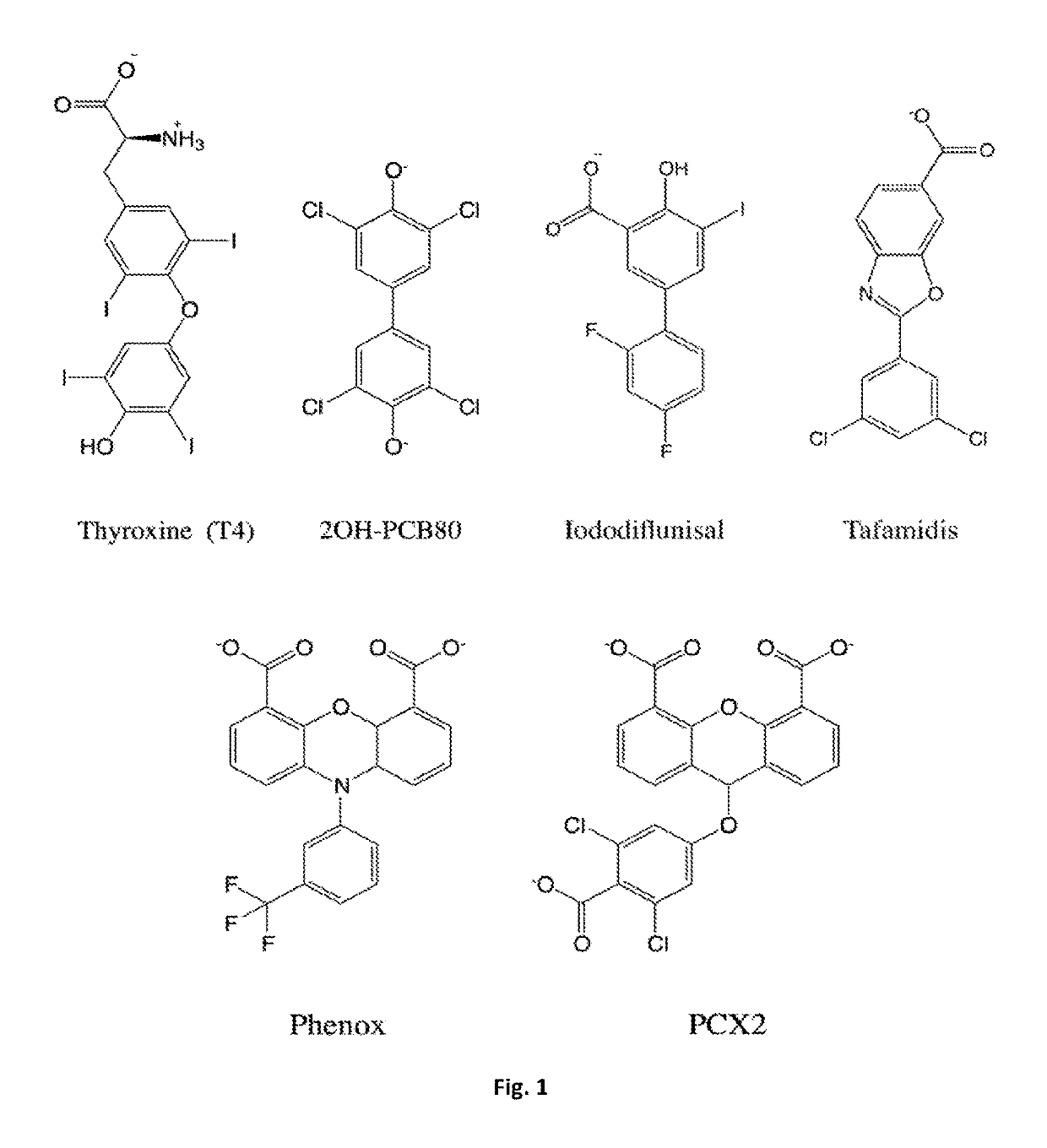
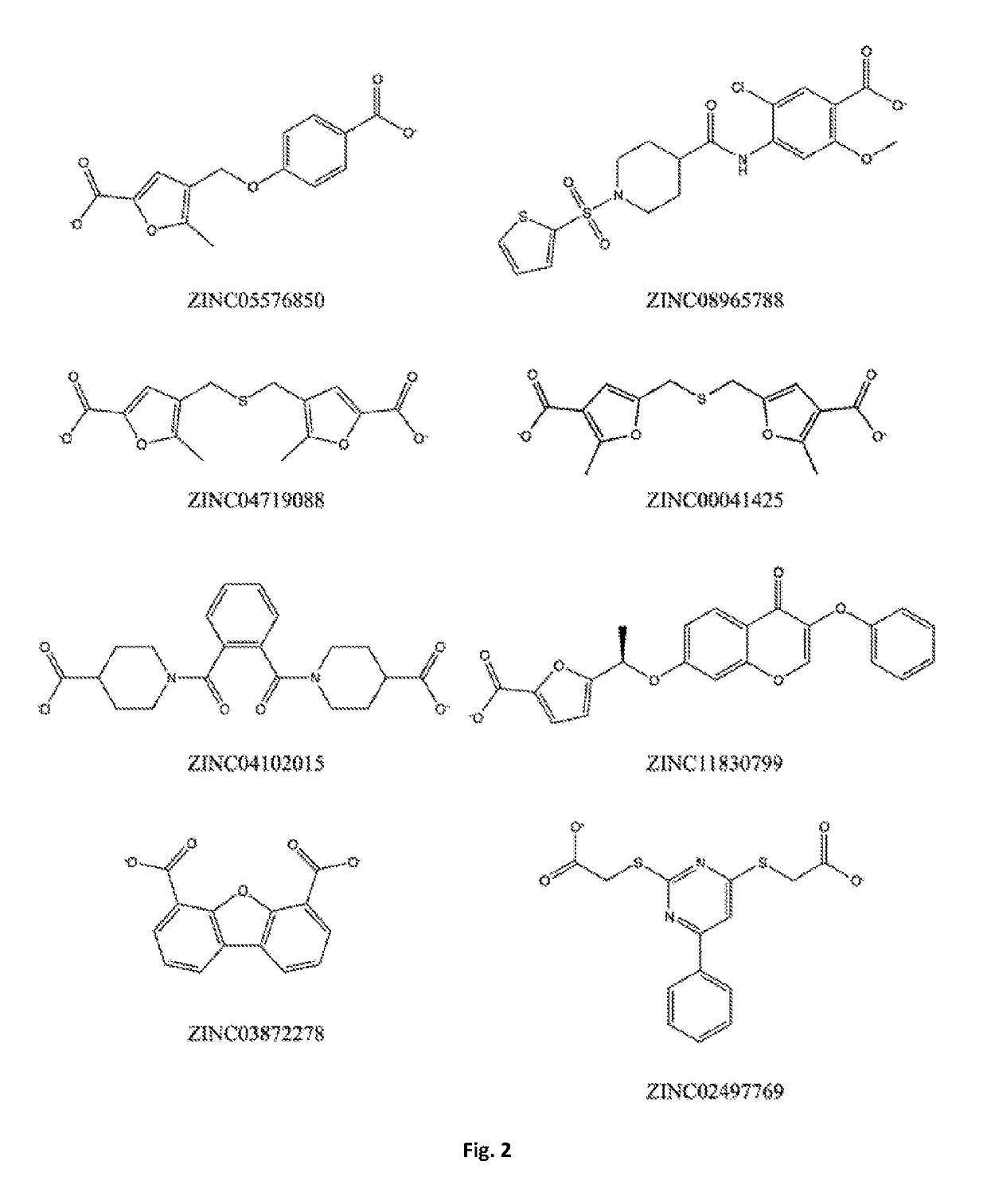
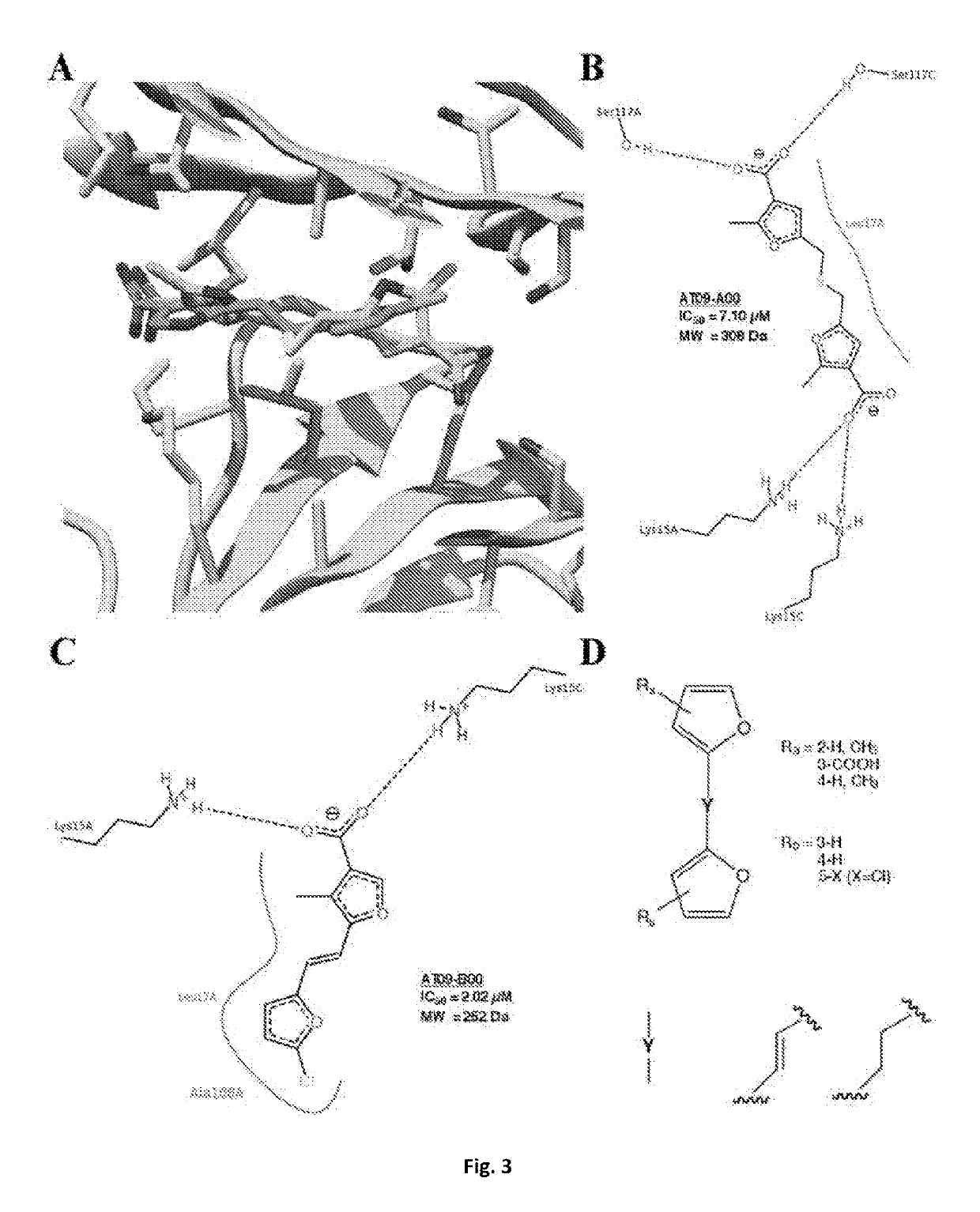
Bis-furan derivatives as transthyretin (TTR) stabilizers and amyloid inhibitors for the treatment of familial amyloid polyneuropathy (FAP)
ActiveUS10377729B2Reduce deliveryImprove stabilityOrganic active ingredientsNervous disorderFuranDisease
The design and synthesis of a novel bis-furan scaffold tailored for high efficiency at inhibiting transthyretin amyloid formation is reported. In vitro results show that the discovered compounds are more efficient inhibitors of amyloid formation than tafamidis, a drug currently used in the treatment of familial amyloid polyneuropathy (FAP), despite their lower molecular weight and lipophilicity. Moreover, ex vivo experiments with the strongest inhibitor in the series, conducted in human blood plasma from normal and FAP Val30Met-transthyretin carriers, disclose remarkable affinity and selectivity profiles. The promises and challenges facing further development of this compound are discussed under the light of increasing evidence implicating transthyretin stability as a key factor not only in transthyretin amyloidoses and several associated co-morbidities, but also in Alzheimer's disease.
Owner:BSIM2 BIOMOLECULAR SIMULATIONS SA
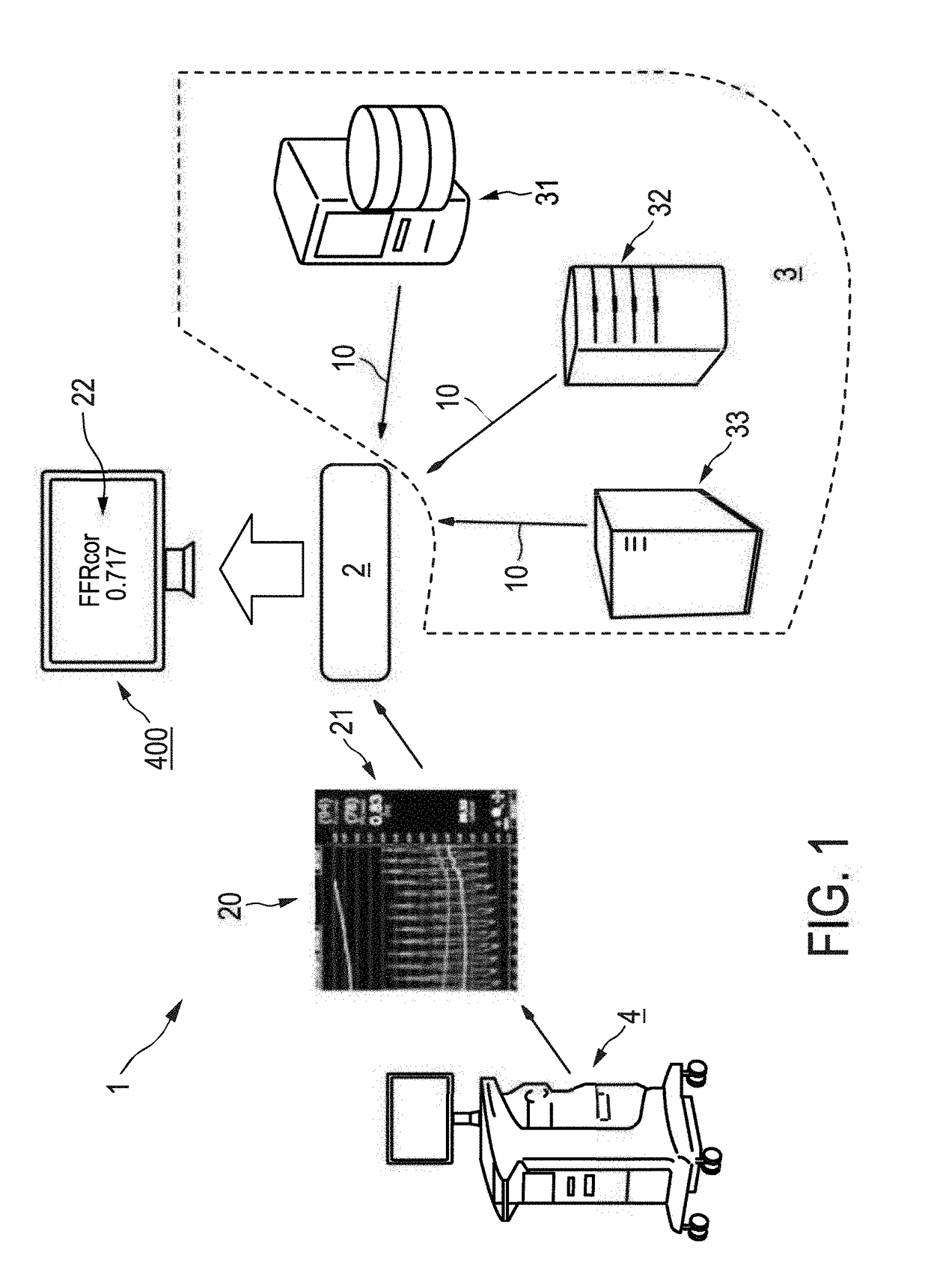
Using archived patient data to correct intravascular measurements for patient co-morbidities
An apparatus is provided which improves the evaluation of a patient's vasculature by applying a correction to invasively acquired intravascular measurement data of a vessel of interest on the basis of archived patient data of said patient from a patient database. By correcting the measurement data, co-morbidities of the patient which may influence the intravascular measurement results are accounted for.
Owner:KONINKLJIJKE PHILIPS NV
Compositions and methods for detecting Anti-endothelial cell antibodies in allograft rejection
ActiveUS20170276687A1Increased riskReduce riskDisease diagnosisBiological testingEnd stage diseaseAllogeneic graft
Provided herein are methods, compositions, and kits for diagnosing allograft rejection of organ transplants by identifying the presence of anti-endothelial cell antibodies. Such methods and compositions are independent of external confounders such as recipient age, transplant center, assay, cause of end-stage disease, co-morbidities, immunosuppression usage, and the like.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Multimarker panel for diabetes type 1 and 2
The present invention relates to a method and means for diagnosing or risk stratification of co-morbidities, particularly cardiovascular complications in diabetes patients. The invention particularly relates to a method for diagnosing whether a diabetes patient is suffering from a cardiovascular complication or is at risk of suffering from a cardiovascular complication, said method comprising the steps of (a) measuring, preferably iii vitro, the level(s) of at least one cardiac hormone or a variant thereof in a sample from the patient, and (b) diagnosing the cardiovascular complication or the risk of suffering from cardiovascular complication by comparing the measured level(s) to known level(s) associated with the cardiovascular complication or risk. The present invention also relates to combining the measurement of markers comprising cardiac hormones, natriuretic peptides, inflammation-associated markers, angiogenesis markers, and markers for platelet activation. Preferred markers are brain natriuretic peptides (particularly NT-proBNP), PIGF, and sCD40L.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Method of Detecting Active Tuberculosis Using Minimal Gene Signature
InactiveUS20190323065A1High sensitivity/specificityReduce in quantityMicrobiological testing/measurementCo morbidityLow resource
A method of detecting active TB in the presence of a complicating factor, for example, latent TB and / or co-morbidities, such as those that present similar symptoms to TB. The disclosure also relates to a minimal gene signature employed in the said method and to a bespoke gene chip for use in the method. The disclosure further relates to use of gene chips and primer sets in the methods of the disclosure and kits comprising the elements required for performing the method. The disclosure also relates to use of the method to provide a composite expression score which can be used in the diagnosis of TB, particularly in a low resource setting.
Owner:IMPERIAL COLLEGE OF SCI TECH & MEDICINE
Methods and devices for removing omental tissue
InactiveUS9211154B2Reduce morbidityEliminates collateral effectBlunt dissectorsExcision instrumentsCo morbidityTissue material
The invention relates to a method of treating obesity, insulin resistance and co-morbidities of these conditions by removing tissue from the abdomen. More specifically, it relates to a method of removing abdominal fat and omentum to which the fat is attached, in order to improve health. The invention includes a device for safely removing this tissue material
Owner:LAUFER MICHAEL D
Stable, water-insoluble R-(+)-α-lipoic acid salt useful for the treatment of diabetes mellitus and its co-morbidities
The present invention relates to oral nutritional and therapeutic products which are useful for preventing or treating compensated and decompensated insulin resistance and associated diseases and sequelae, or diabetes mellitus and its sequelae, complications, and co-morbidities, comprising magnesium R-(+)-alpha-lipoate.
Owner:BIOLINK LIFE SCI
Traditional Chinese medicine decoction containing acronychia pedunculata and its application in treating tumor drugs
InactiveCN110123864AImprove immunityTumor suppressionDispersion deliveryAntineoplastic agentsAcronychia pedunculataTumor-Associated Fibroblasts
The invention belongs to the technical field of medicine preparation, and particularly relates to a traditional Chinese medicine decoction for treating tumors and an application thereof. In the application of the present invention, acronychia pedunculata and the components contained in a compounded medical and edible plant can improve the overall immunity, can change the tumor microenvironment (TEM), and activates a part of the subgroups in tumor-associated fibroblasts (CAF) which inhibit tumors, and the decoction synergistically play a role in inhibiting tumors and preventing recurrence and metastasis. The clinical data confirms that the composition can effectively inhibit tumors, improves clinical symptoms, and prevents co-morbidity and complications.
Owner:海南长永恒生物科技有限公司
Methods and devices for removing omental tissue
InactiveUS20140188112A1Avoid bleedingReduce morbidityMedical devicesNon-surgical orthopedic devicesCo morbidityTissue material
The invention relates to a method of treating obesity, insulin resistance and co-morbidities of these conditions by removing tissue from the abdomen. More specifically, it relates to a method of removing abdominal fat and omentum to which the fat is attached, in order to improve health. The invention includes a device for safely removing this tissue material
Owner:LENR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com