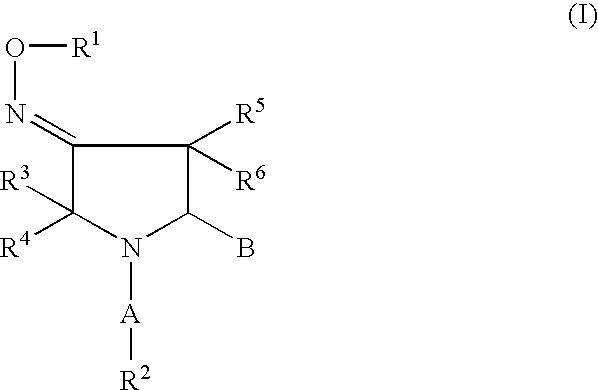
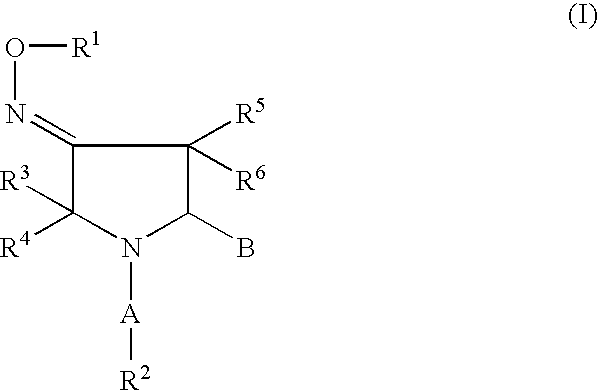
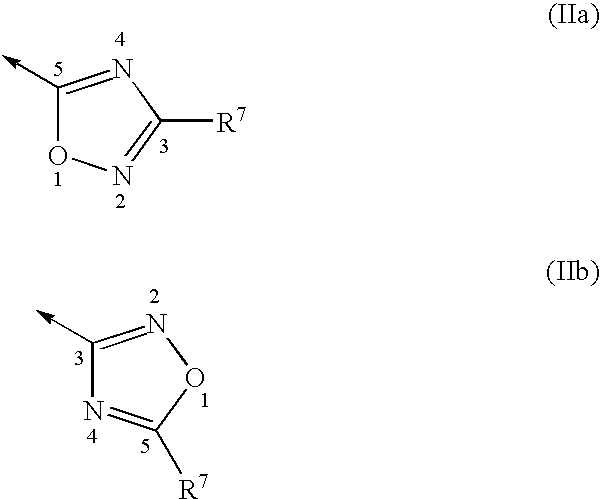
Pyrrolidine oxadiazole-and thiadiazole oxime derivatives being oxytocin receptor antagonists
a technology of oxytocin receptor and oxime, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of inconvenient agents, respiratory depression and cardiac arrest, and preterm labor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for the Solution-Phase Synthesis of Pyrrolidine Oxadiazole Derivatives of General Formula I, With B=IIa (Schemes 1,11): (3EZ,5S)-1-([1,1′-biphenyl]-4-ylcarbonyl)-5-(3-methyl-1,2,4-oxadiazol-5-yl)-3-pyrrolidinone O-methyloxime, (3E,5S)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-5-(3-methyl-1,2,4-oxadiazol-5-yl)-3-pyrrolidinone O-methyloxime and (3Z,5S)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-5-(3-methyl-1,2,4-oxadiazol-5-yl)-3-pyrrolidinone O-methyloxime
[0289]
a) Protocol for the Formation of the Oxadiazole Ring
[0290] Diisopropylcarbodiimide (3.16 g, 25.17 mmol) was added to a solution of (2S,4EZ)-1-(tert-butoxycarbonyl)-4-(methoxyimino)-2-pyrrolidine-carboxylic acid (Intermediate 2, 6.50 g, 25.17 mmol) and acetamidoxime (Intermediate 7, 1.86 g, 25.17 mmol) in DCM (55 ml) and stirred overnight at room temperature (DCM-insoluble amidoximes were pre-dissolved in 15 THF, to which was added a solution of DIC and Intermediate 2 in DCM). After filtering at the pump...
example 2
(3EZ,5S)-1-[(2′-chloro[1,1′-biphenyl]-4-yl)carbonyl]-5-(3-methyl-1,2,4-oxadiazol-5-yl)-3-pyrrolidinone O-methyloxime
[0298]
[0299] Following the general methods as outlined in Example 1 (Method B), starting from (2S,4EZ)-1-(tert-butoxycarbonyl)-4-(methoxyimino)-2-pyrrolidine-carboxylic acid (Intermediate 2), N′-hydroxyethanimidamide (Intermediate 7) and 2′-chloro [1,1′-biphenyl]-4-carboxylic acid (Intermediate 10), the title compound was isolated, after flash-chromatography, as a mixture of E- / Z-isomers as an oil in 31% yield (98.5% purity by HPLC).
[0300] Oil; 1H NMR (300 MHz, CDCl3): 2.41 (s, 3H, CH3), 2.96-3.31 (m, 2H, CH2), 3.87 (s, 3H, NOCH3), 4.31-4.59 (m, 2H, CH2), 6.03 (m, 1H), 7.30 (s, 3H, H arom.), 7.50-7.64 (m, 5H, H arom.); MS(ESI+): 411.2; MS(ESI−): 408.9.
example 3
(3EZ,5S)-5-(3-methyl-1,2,4-oxadiazol-5-yl)-1-{[2′-(trifluoromethyl)[1,1′-biphenyl]-4-yl]carbonyl}-3-pyrrolidinone O-methyloxime
[0301]
[0302] Following the general methods as outlined in Example 1 (Method B), starting from (2S,4EZ)-1-(tert-butoxycarbonyl)-4-(methoxyimino)-2-pyrrolidine-carboxylic acid (Intermediate 2), N′-hydroxyethanimidamide (Intermediate 7) and 2′-(trifluoromethyl) [1,1′-biphenyl]-4-carboxylic acid (Intermediate 10), the title compound was isolated, after flash-chromatography, as a mixture of E- / Z-isomers as an oil in 44% yield (88.2% purity by HPLC).
[0303] Oil; 1H NMR (300 MHz, CDCl3): 2.40 (s, 3H, CH3), 2.88-3.31 (m, 2H, CH2), 3.87 (s, 3H, NOCH3), 4.27-4.53 (m, 2H, CH2), 6.03 (m, 1H), 7.27-7.70 (m, 7H, H arom.), 7.77 (m, 1H, H arom.); MS(ESI+): 445.4; MS(ESI−): 443.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com