Conjugated psychotropic drugs and uses thereof
a psychotropic drug and chemical conjugate technology, applied in the field of conjugated psychotropic drugs, can solve the problems of limiting their use, increasing body weight, mood disturbance, etc., and achieve the effect of reducing adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0225] Reference is now made to the following examples, which together with the above descriptions, illustrate the invention in a non limiting fashion.
Chemical Syntheses and Analyses
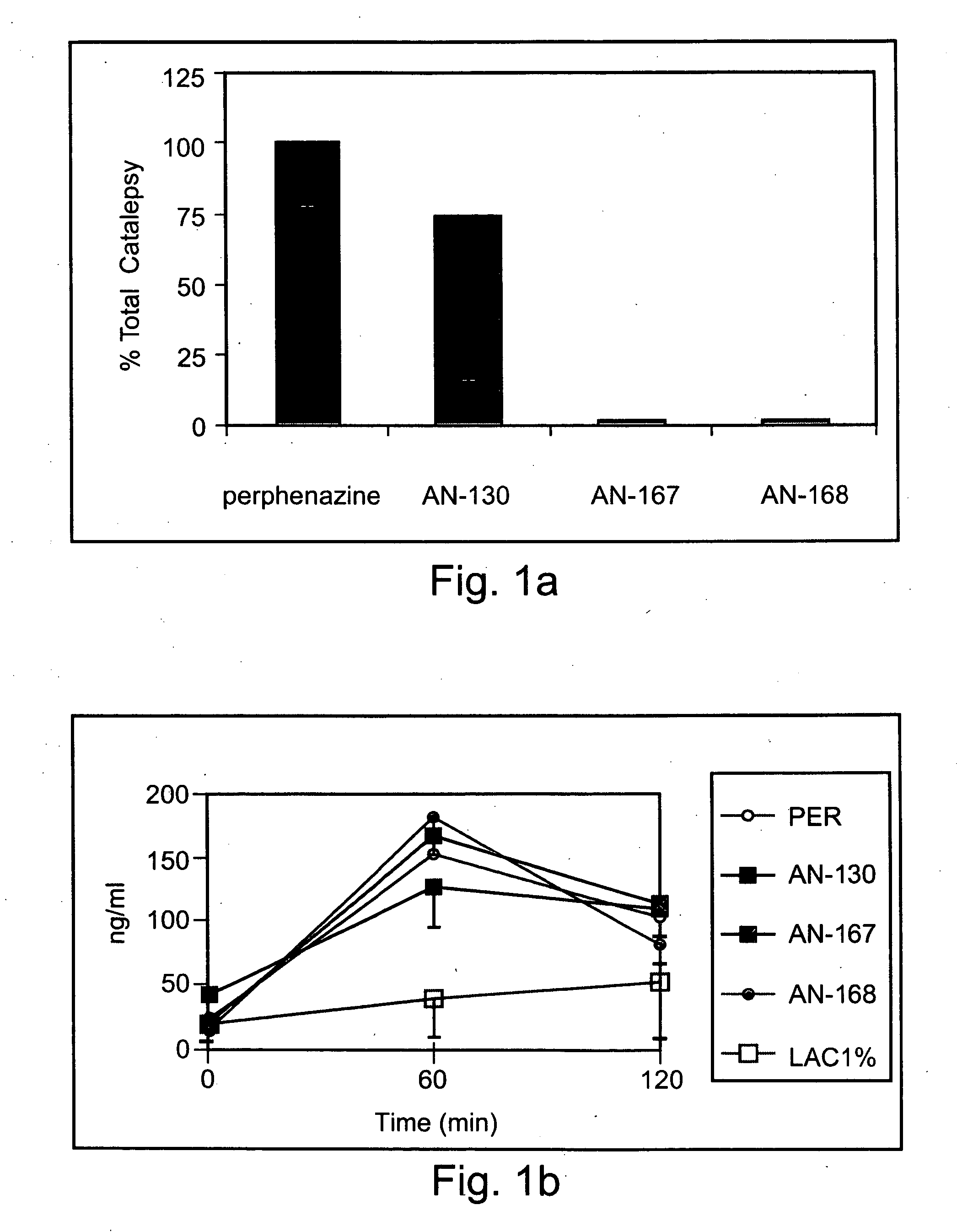
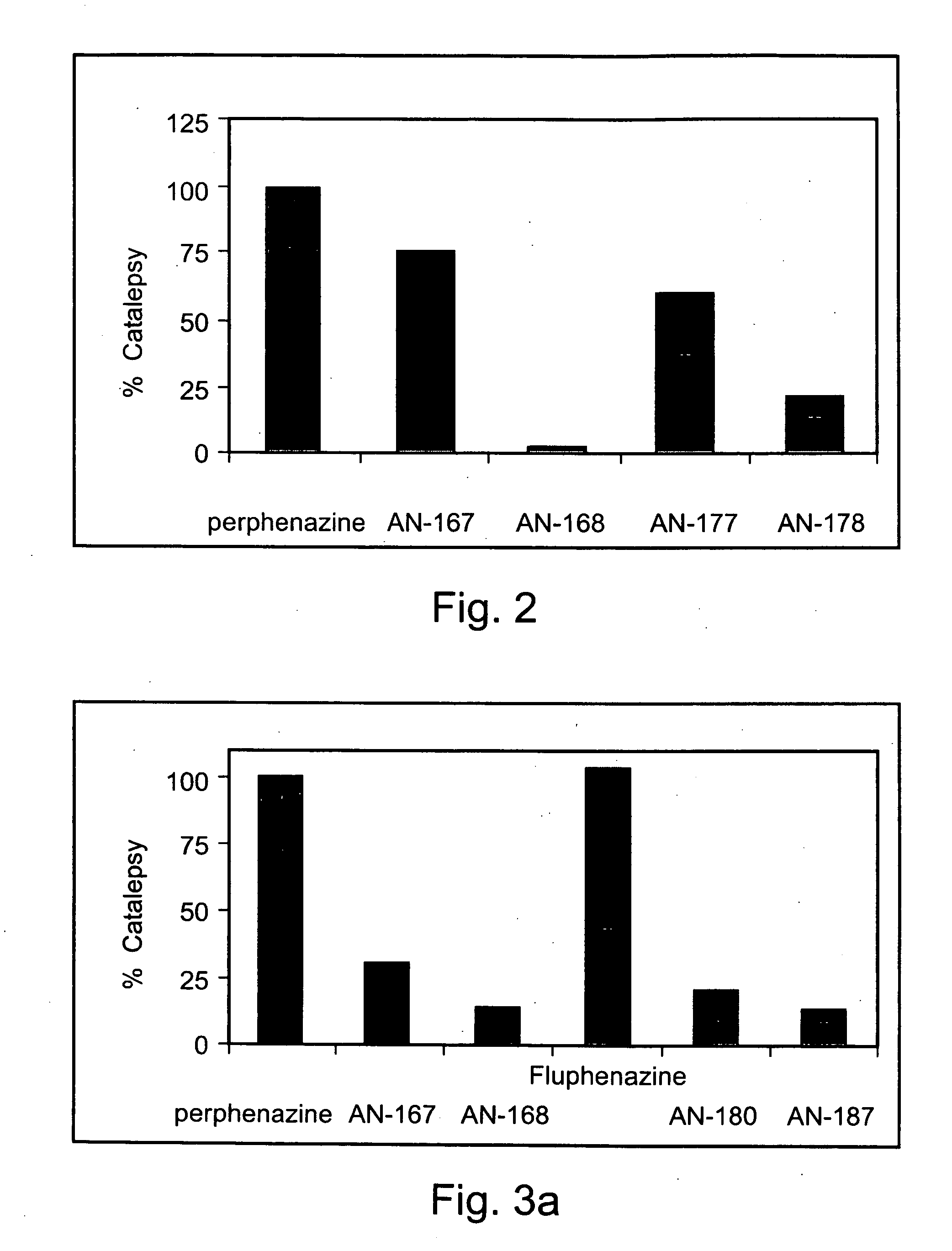
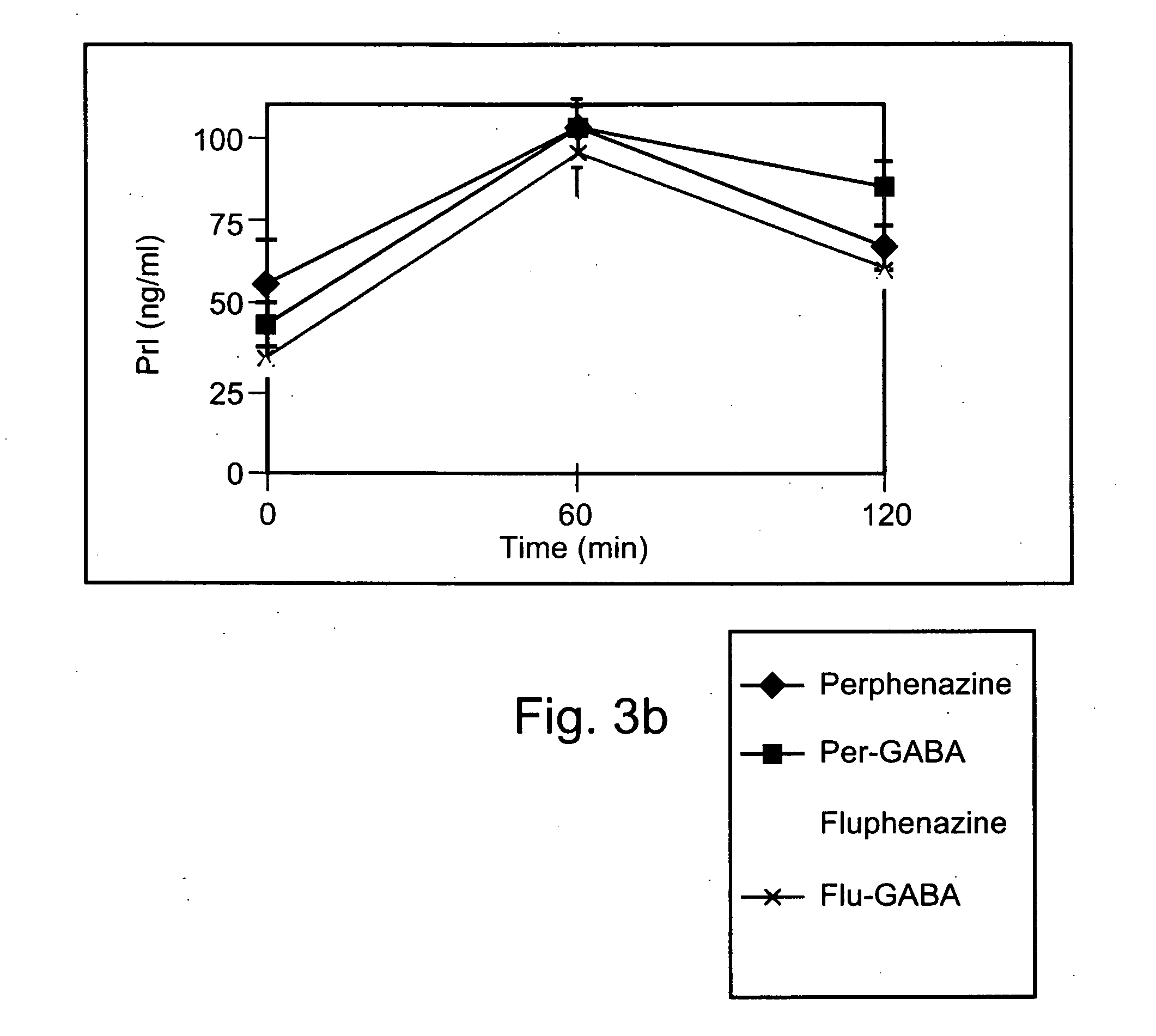
[0226] Exemplary chemical conjugates of the present invention were synthesized by reacting the psychotropic agents perphenazine, fluphenazine and valproic acid with the short-chain fatty acids propionic acid, butyric acid and valeric acid and / or with 4-phenylbutyric acid and γ-aminobutyric acid (GABA). The compounds were prepared in high yields and were isolated as crystalline solids, soluble in aqueous 1% lactic acid.
[0227] Synthesis of chemical conjugates prepared from perphenazine or fluphenazine and an organic acid—General Procedure: A mixture of the neuroleptic agent perphenazine or fluphenazine (1 equivalent), an acyl chloride derivative of a short-chain fatty acid (1.1 equivalents) and, optionally, Et3N (2 equivalents) (used to free starting materials found as their HCl salts) in 5-10 ml dimeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com