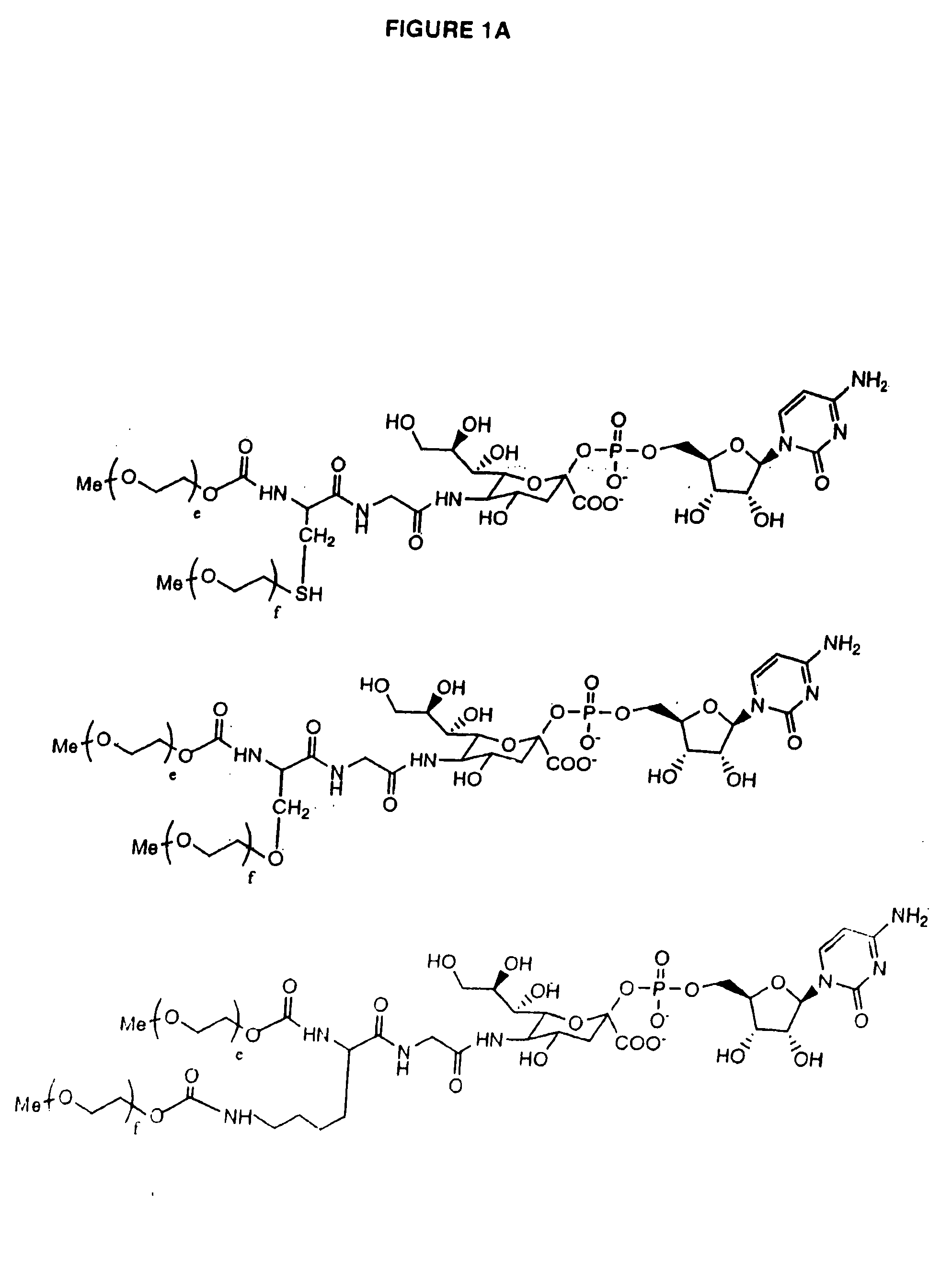
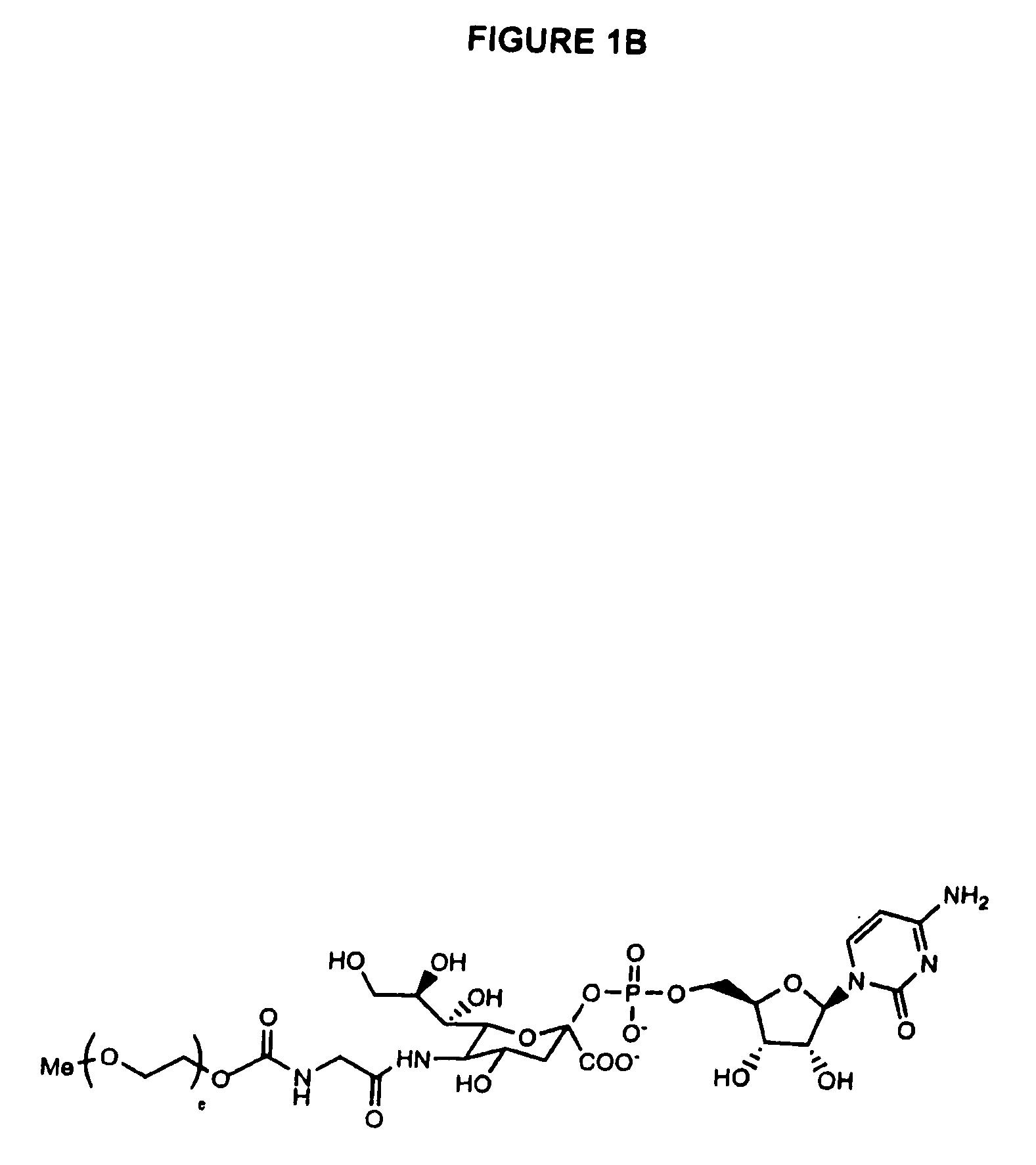
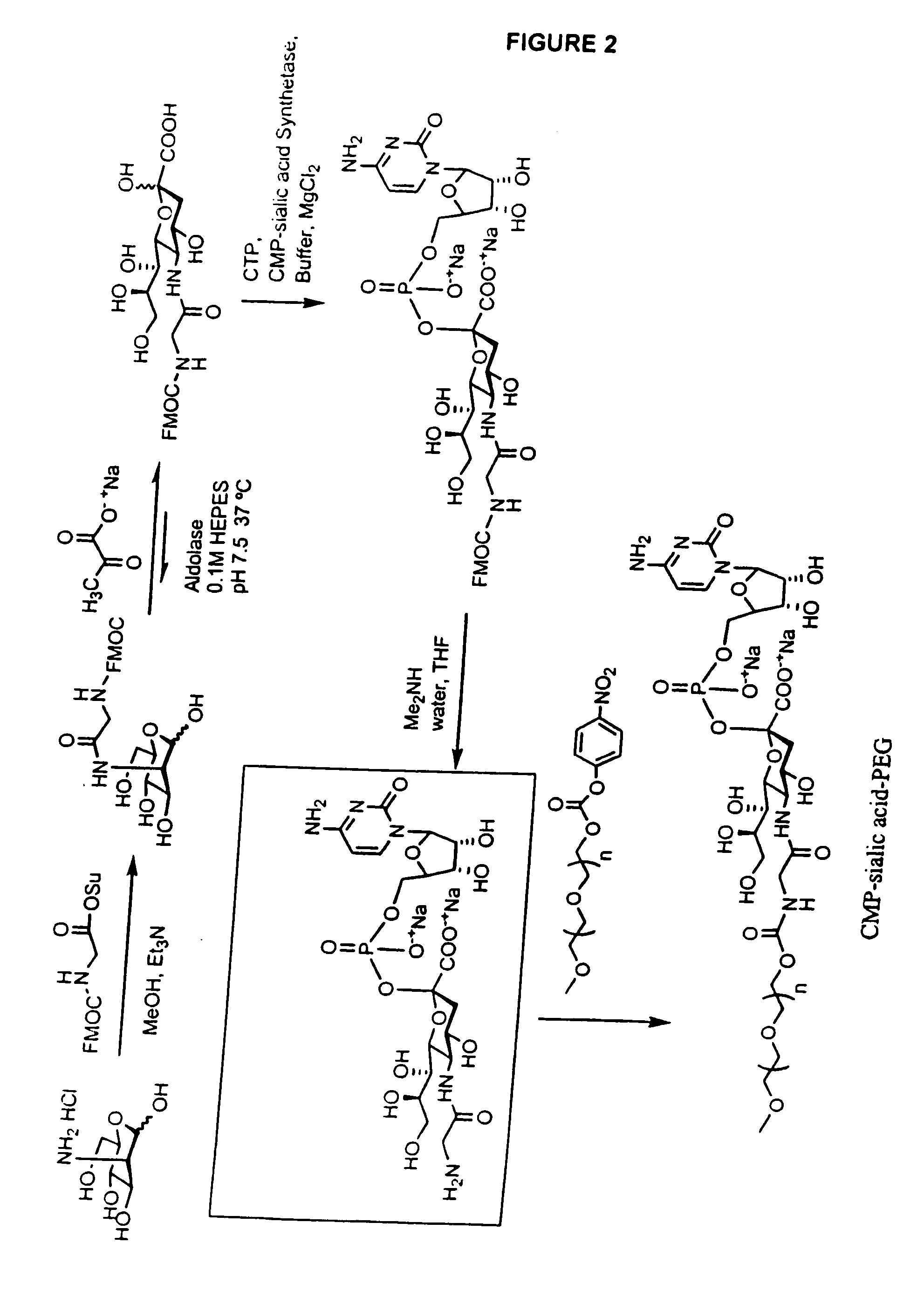
One pot desialylation and glycopegylation of therapeutic peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of PEGylated Factor VIIa.
[0464] GlycoPEGylated samples of Factor VIIa were purified with a modified anion-exchange method. Samples were handled at 5° C. Immediately before loading the column, 1 g Chelex 100 (BioRad) per 10 mL Factor VIIa solution was added to the remodeled sample. After stirring for 10 min, the suspension was filtered on a cellulose acetate membrane (0.2 μm) with a vacuum system. The retained chelator resin on the filter was washed once with 1-2 mL water per 10 mL bulk. The conductivity of the filtrate was adjusted to 10 mS / cm at 5° C., and adjusted to pH 8.6, if necessary.
[0465] Anion exchange was performed at 8-10° C. A column containing Q Sepharose FF was prepared before loading by washing with 1 M NaOH (10 column volumes), water (5 column volumes), 2 M NaCl, 50 mM HOAc, pH 3 (10 column volumes), and equilibrating with 175 mM NaCl, 10 mM glycylglycine, pH 8.6 (10 column volumes). For each PEGylation reaction, 15-20 mg Factor VIIa was loaded on to ...
example 2
Determination of PEGylated Isoforms by Reversed Phase HPLC Analysis.
[0469] PEGylated Factor VIIa was analyzed by HPLC on a reversed-phase column (Zorbax 300SB-C3, 5 μm particle size, 2.1×150 mm). The eluants were A) 0.1 TFA in water and B) 0.09% TFA in acetonitrile. Detection was at 214 nm. The gradient, flow rate, and column temperature depended on the PEG length (40 KDa, 20 KDa, and 10 KDa PEG: 35-65% B in 30 min, 0.5 mL / min, 45° C.; 10 KDa PEG: 35-60% B in 30 min, 0.5 mL / min, 45° C.; 5 KDa: 40-50%B in 40 min, 0.5 mL / min, 45° C.; 2 KDa: 38-43% B in 67 min, 0.6 mL / min, 55° C.). The identity of each peak was assigned based on two or more of four different pieces of evidence: the known retention time of native Factor VIIa, the SDS-PAGE migration of the isolated peak, the MALDI-TOF mass spectrum of the isolated peak, and the orderly progression of the retention time of each peak with increasing number of attached PEG.
example 3
Determination of Site of PEG Attachment by Reversed-phase HPLC.
[0470] Factor VIIa and PEGylated Factor VIIa variants were reduced by mixing sample (10 μL at a concentration of 1 mg / mL) with reducing buffer (40 μL, 50 mM NaCl, 10 mM glycylglycine, 15 mM EDTA, 8 M urea, 20 mM DTT, pH 8.6) for 15 min at room temperature. Water (50 μL) was added and the sample cooled to 4° C. until injected on the HPLC (<12 hrs). The HPLC column, eluants, and detection were as described above for non-reduced samples. The flow rate was 0.5 mL / min and the gradient was 30-55% B in 90 min, followed by a brief wash cycle up to 90% B. The identity of each peak was assigned as described in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com