Modulation of Abeta levels by beta-secretase BACE2
a technology of beta-secretase and abeta, which is applied in the field of identification and use of amyloid (a) level modulators, can solve the problem of weaker activity than -secretase activity, and achieve the effect of suppressing a production and promoting the alternative non-amyloidogenic app processing pathway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods Used in the BACE2 Assays
[0108] 1. Cloning and Coexpression of BACE and BACE2
[0109] BACE 2 was amplified from human brain cDNAs by Taq Polymerase Chain Reaction (PCR) using sequence information from PCT publication W099 / 34004-A2. The amplified PCR product was cloned into pCRII-TOPO vector using TOPO TA cloning techniques (Invitrogen Co., San Diego, Calif.). Following restriction enzyme digestion, the BACE2 DNA fragment was sub-cloned into Not I and BamH I sites of mammalian expression vector pcDNA 3.1(−). Restriction enzyme digestion pattern and sequence data confirmed the presence and orientation of the insert in pCDNA3.1(−) / BACE2. BACE was similarly cloned from human brain cDNAs using sequence information available from Vassar et al., [1999] ibid) to generate a mammalian expression vector pCDNA3.1 (−) / BACE.
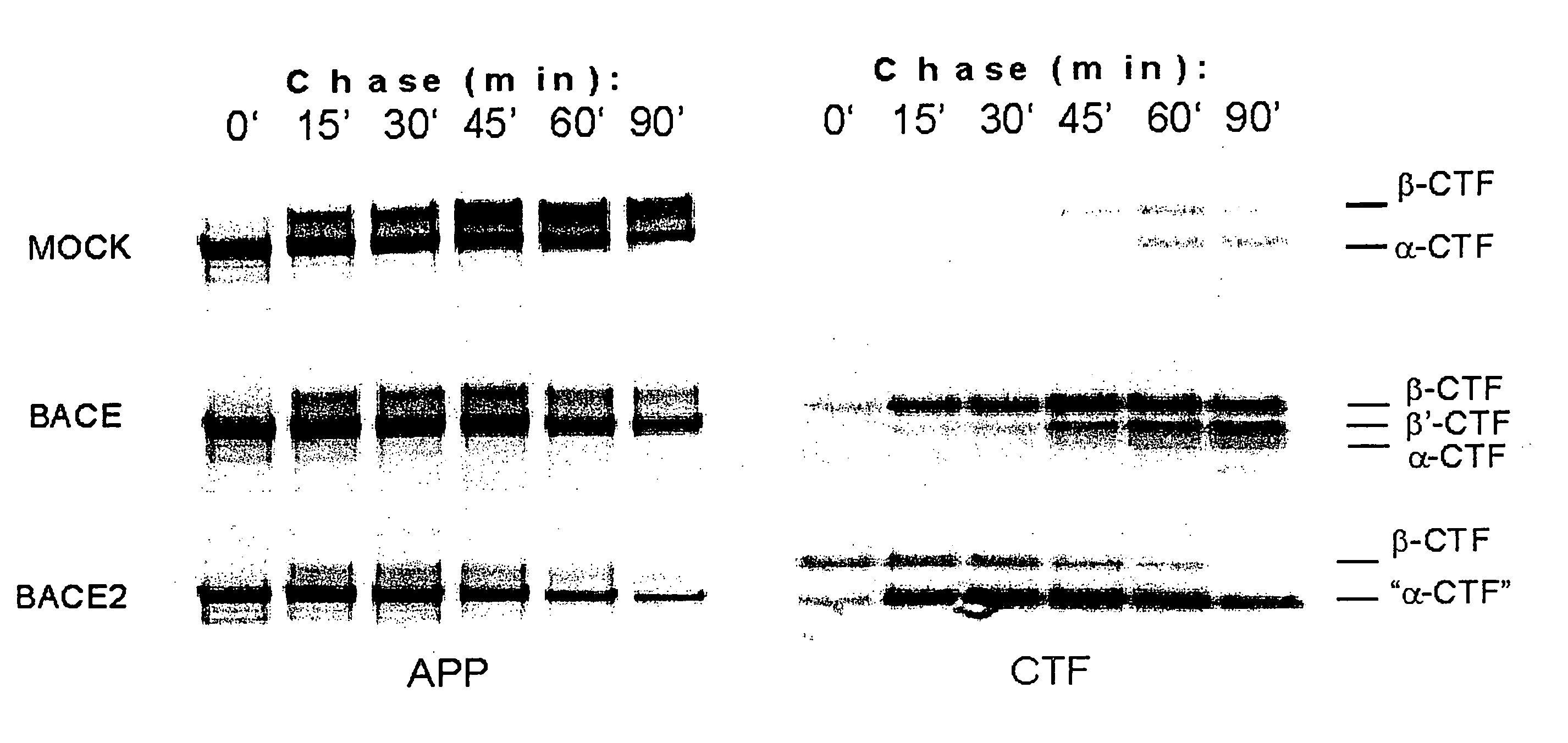
[0110] Expression vectors containing BACE and BACE2 cDNAs were transfected into HEK293T cells in the presence of APP751sw or CT100 (Shoji et al., Scienc...
example 2
BACE2 Possesses β-secretase Activity In Vitro
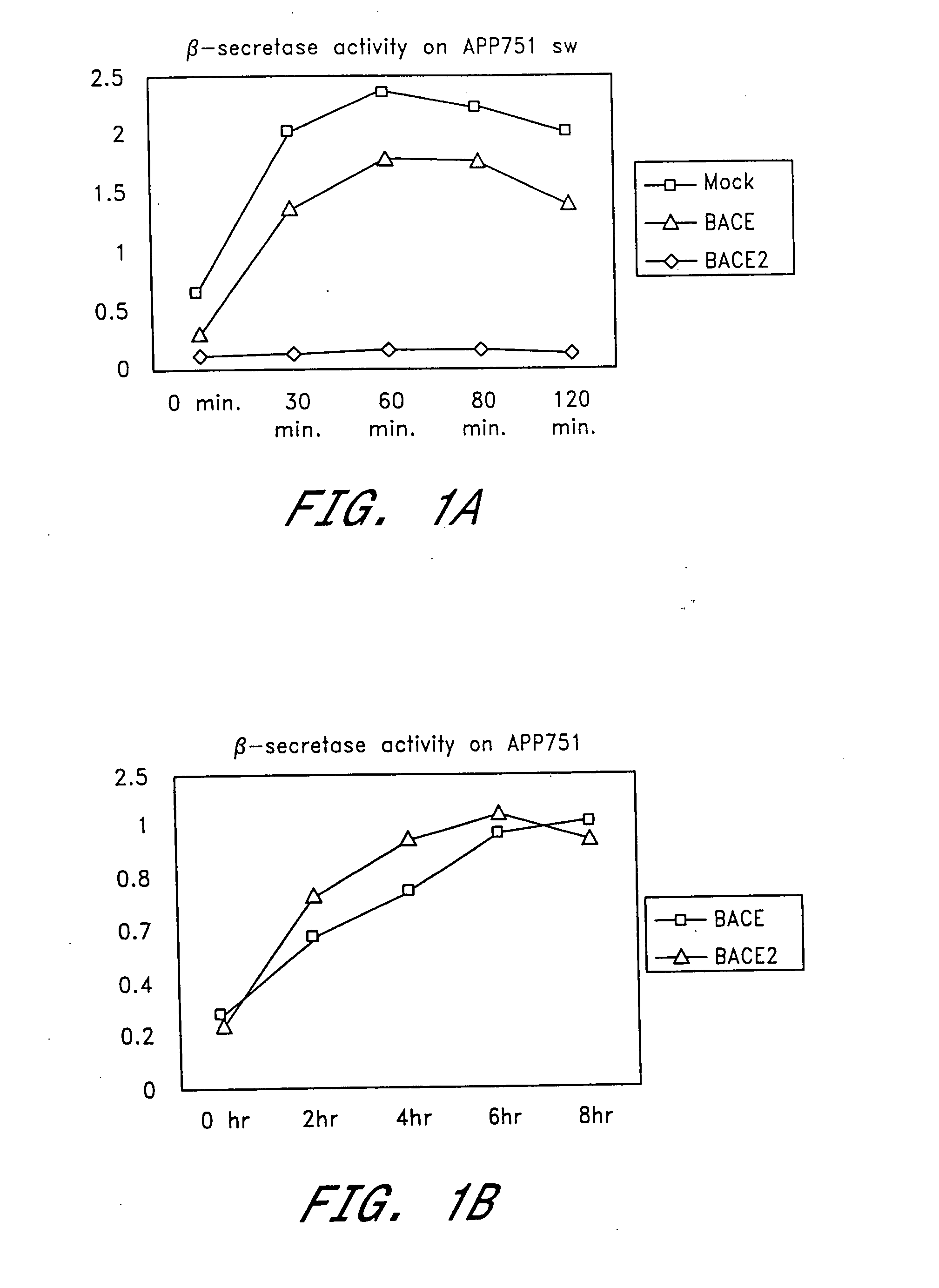
[0136] To determine whether BACE2 possesses β-secretase activity, we reconstituted the cleavage of APP751sw and APP751 wt at the β-secretase site in a cell-free assay. Equal amounts of membrane extracts from either mock, BACE or BACE2 transfected HEK293 cells were incubated with partially purified full length APP751sw or APP751wt for the indicated times. The formation of β-NTF was then determined using the respective β-NTF specific ELISA as described in General Methodology.
[0137] Extracts from both BACE and BACE2 transfected cells showed a time-dependent increase in β-NTF formation using APP751sw as a substrate (FIG. 1a). This activity was absent from mock-transfected extracts within the 2 hour incubation time. Endogenous secretase levels as present in the mock-treated extracts required incubation times between 12 and 16 hours in order to detect sufficient β-NTF levels in this assay (data not shown). As a consequence, all detectable β-s...
example 3
BACE2 Overexpression Leads to Suppression of Aβ formation in HEK293T Cells
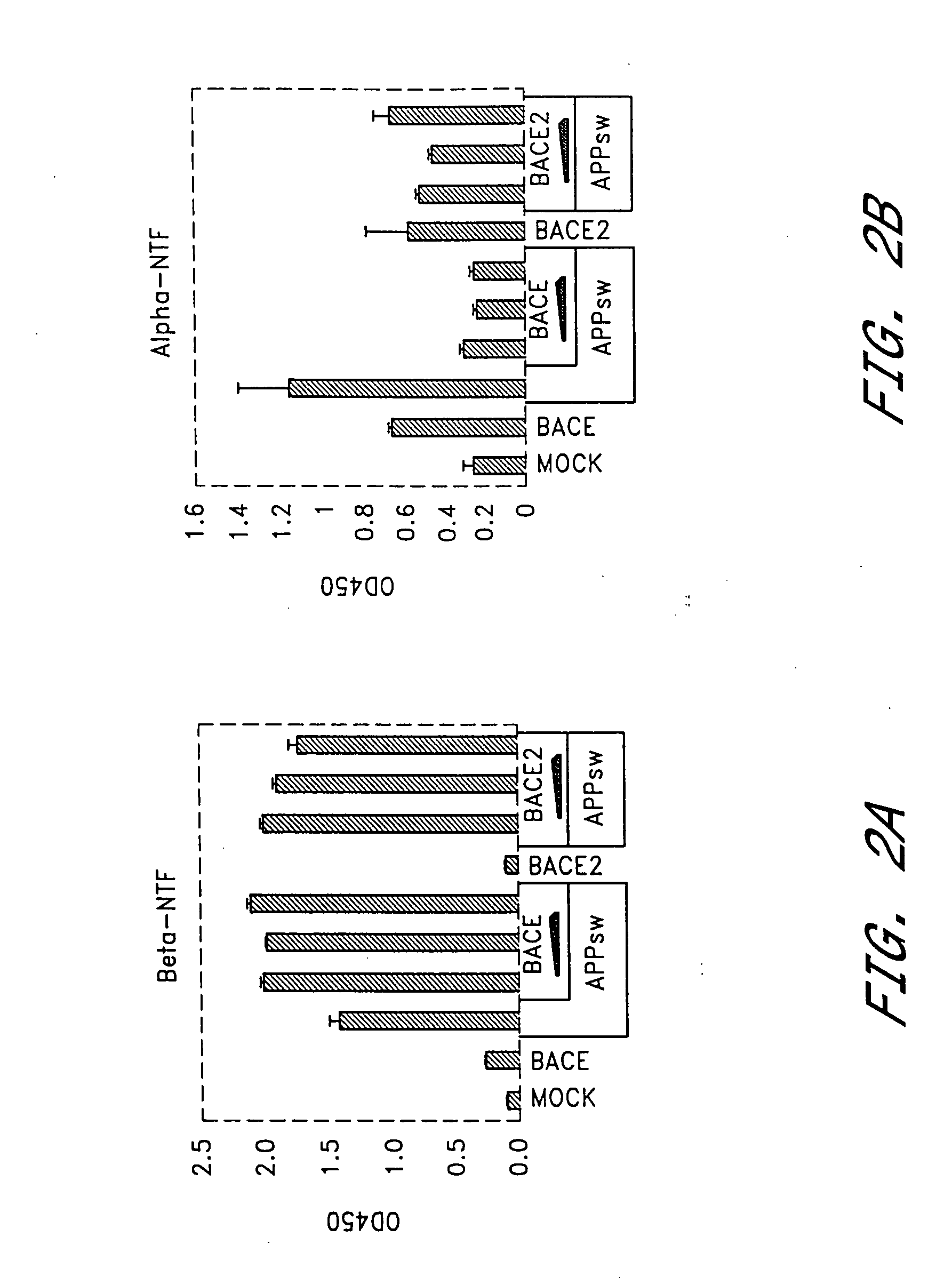
[0139] Given the ability of BACE2 to cleave APP at the β-secretase site in vitro, we investigated the role of BACE2 in HEK293T cells relative to BACE. Variable amounts of BACE2 or BACE expression plasmids were co-transfected with constant amount of DNA encoding APP751sw substrate cDNA. Cell supernatants were collected 48 hours or 72 hours post-transfection and analyzed for α-NTF, β-NTF, total Aβ and Aβ42 as described in General Methodology. Expression of APP751sw alone led to a significant increase in β-NTF, α-NTF, total Aβ and Aβ42 (FIG. 2a-d) compared to mock-transfected cells. This suggested that endogenous secretases were not limiting for β-NTF or Aβ formation under these conditions. When BACE was expressed in addition to APP751sw, β-NTF levels (FIG. 2a) were further increased as expected from earlier reports (Vassar et al,[1999] ibid; Sinha et al., [1999] ibid; Yan et al., [1999] ibid). As a consequence,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com