Compositions for weight management
a weight management and composition technology, applied in the field of compositions for weight management, can solve the problems of increasing the risk of illness, increasing the risk of all-cause death, and at least 300,000 excess deaths, and achieving the effect of preventing or reducing weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
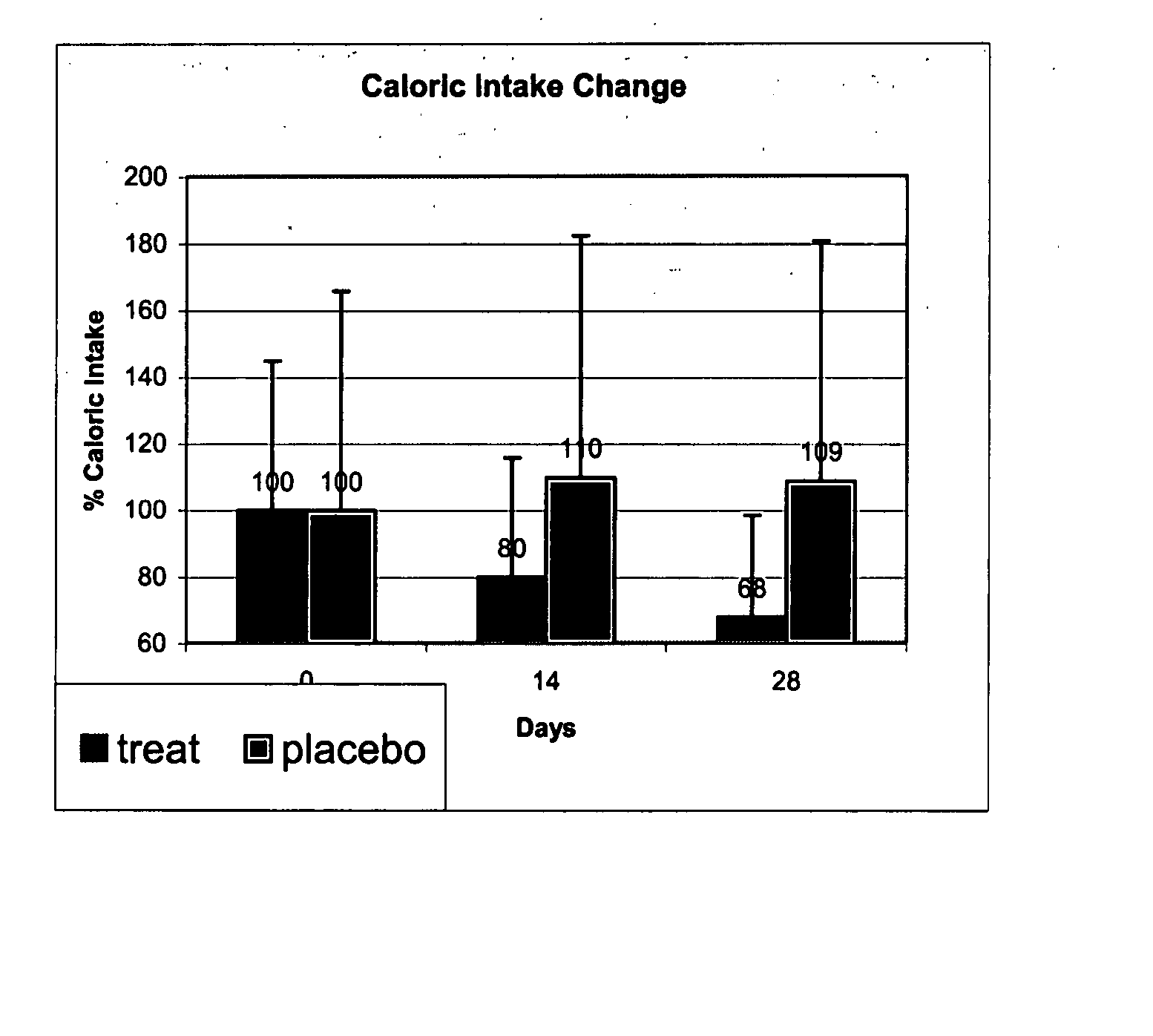
[0301] The following study was conducted to evaluate the effect of oral administration of betahistine on food intake:
[0302] Twenty obese but otherwise healthy persons were recruited. Their characteristics upon recruitment are shown in Table 1. Exclusion criteria for the study were age younger than 18, active diseases, medication use, known hypersensitivity or contra-indication for the use of betahistine.
[0303] Each subject was randomly allocated to receive betahistine 16 mg at 10:00 and 16:00 or placebo. Weight, caloric intake (24 hour recall) and appetite during the day (VAS, Visual Analogue Score) were obtained on day 0, 14 and 28 of the study. Subjects were instructed to eat according to their appetite without limitations.
[0304] Statistical significance was assessed with t-test. BMI stands for body mass index.
TABLE 1Patients' characteristicsTreatmentbetahistinePlaceboAge48 ± 9 38 ± 15NSWeight (kg)93 ± 1790 ± 4NSBMI35.1 ± 7.3 32.7 ± 1.7NSMean caloric intake (kcal)975 ± 47213...
example 2
[0320] In a further study, a healthy, overweight woman was treated twice daily with betahistine 16 mg for a month without any dietary changes. The level of certain metabolites of the woman, as observed in blood tests, was measured before and after the betahistine treatment and are presented in Table 2 below.
TABLE 2Day 0Day 30ChangeTotal cholesterol167155−7%HDL-cholesterol5458+7%LDL-cholesterol9984−15% Triglycerides6962−10% Fructoseamine195202+4%
[0321] As seen in Table 2, the results show that during the 30-day time period studied, the total cholesterol level of the subject decreased, with a corresponding decrease in the level of LDL-cholesterol and an increase in the level of HDL-cholesterol. The level of triglycerides decreased, while the level of fructoseamine increased very slightly. It is therefore concluded that the subject restricted her fat intake, (as shown by the decrease in LDL-cholesterol), without reducing her carbohydrate intake, (as shown by the slight increase in fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmacological half life | aaaaa | aaaaa |
| pharmacological half life | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com