Diabetic nephropathy therapies
a nephropathy and diabetes technology, applied in the field of diabetes nephropathy therapies, can solve the problems of reducing kidney function, hypertension, renal failure, compromising quality of life, etc., and achieve the effect of inhibiting and preventing these processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of Early Stage Features of Diabetic Nephropathy
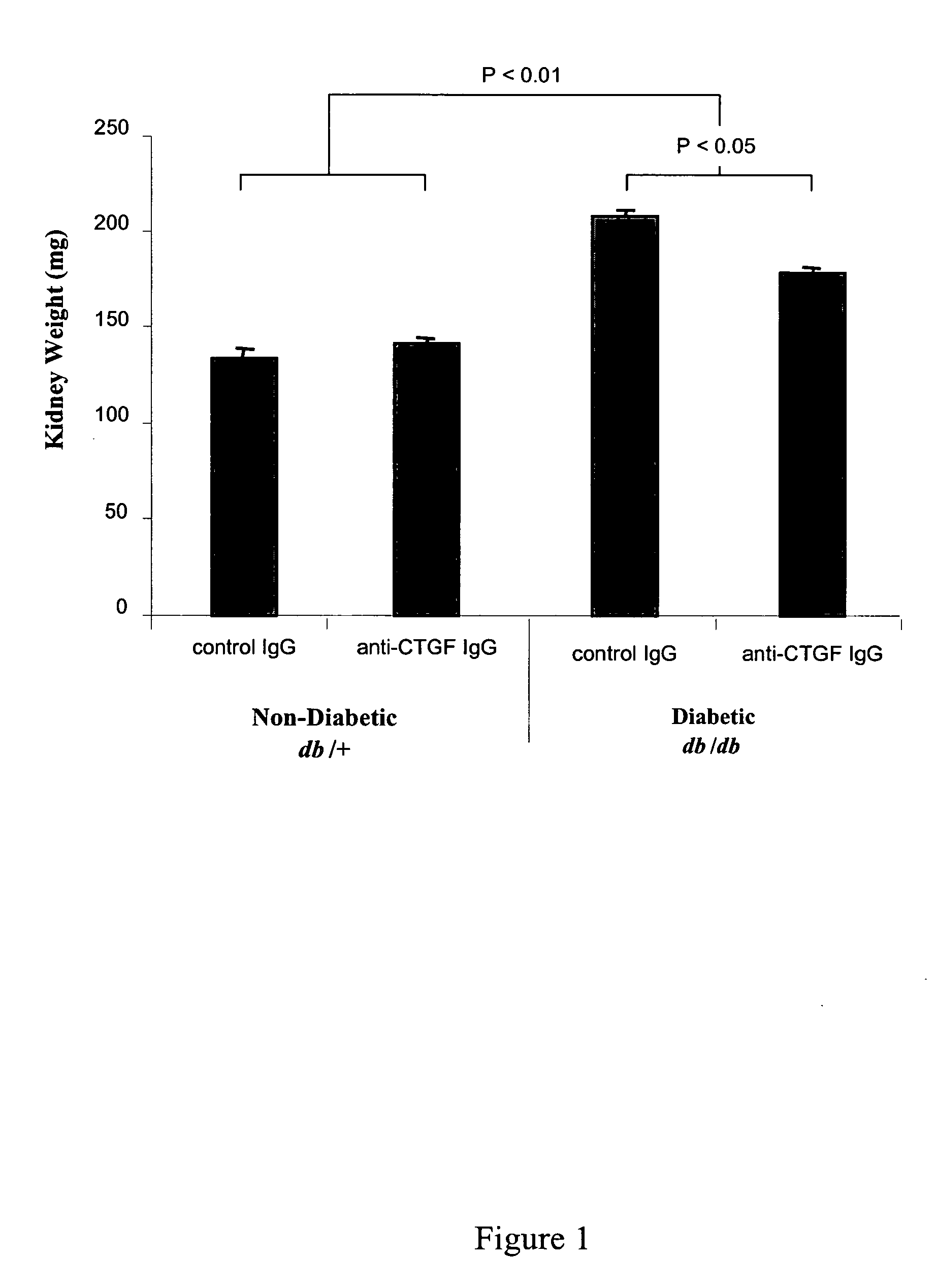
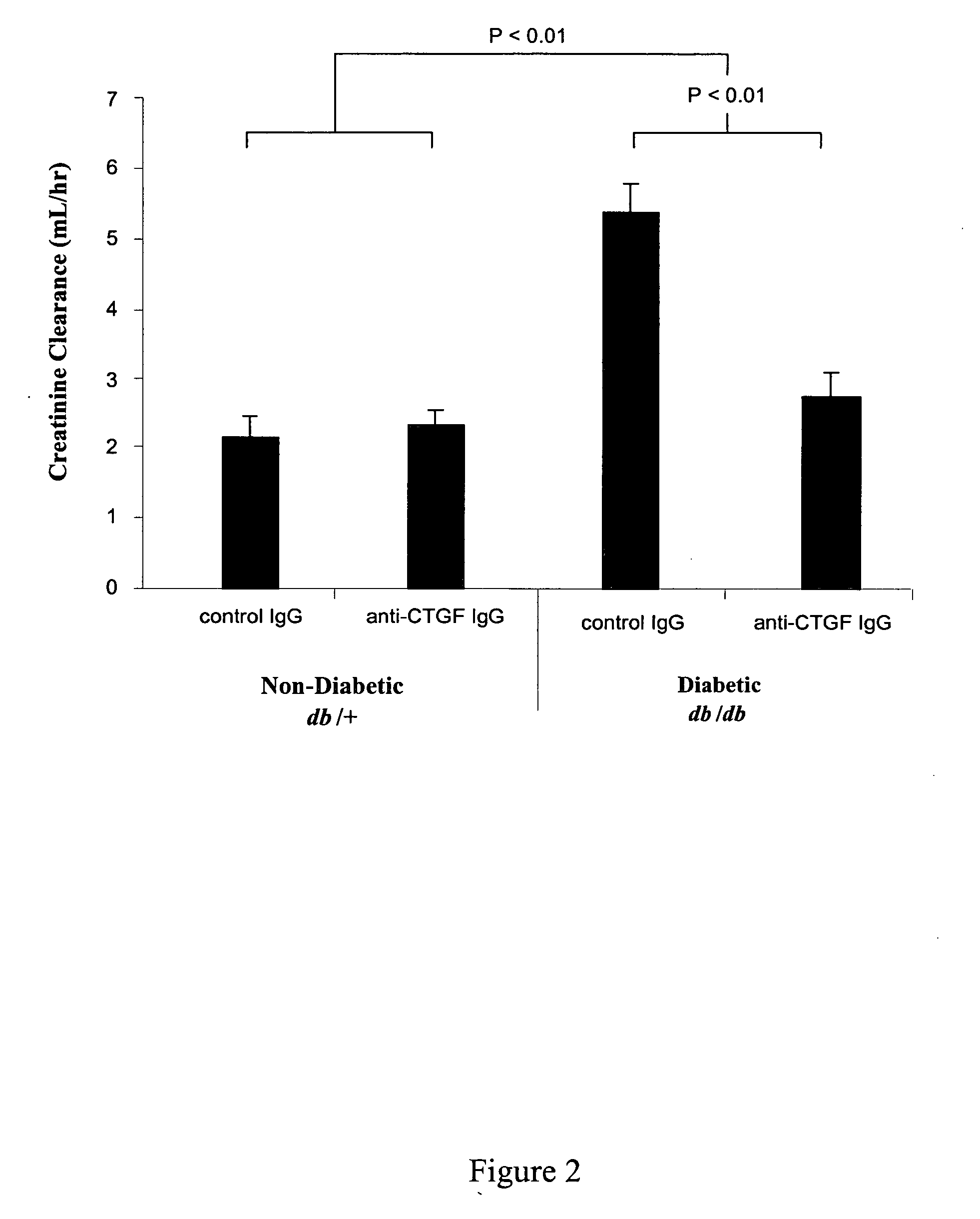
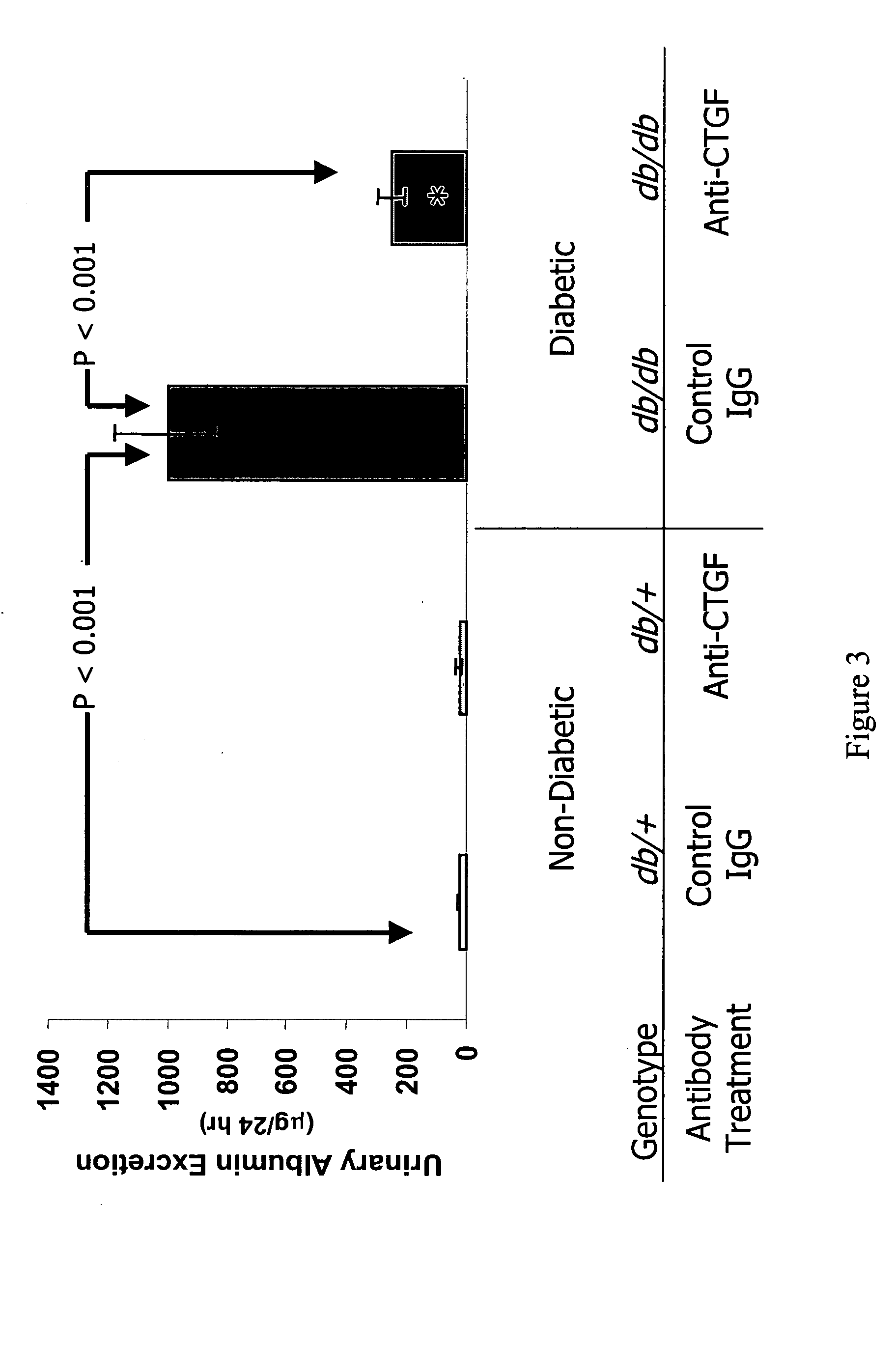
[0109] The methods of the invention were used to demonstrate broad-spectrum efficacy in an animal model for certain aspects of early stage diabetic nephropathy as follows. Eight-week-old mice having a loss-of-function mutation in the leptin receptor (Ob-R; encoded by the db gene) were obtained from Harlan, Indianapolis Ind. These db / db mice serve as an animal model of obese type 2 diabetes, and, in particular, a model of obese type 2 diabetic nephropathy characterized by early aspects of diabetic nephropathy, including, for example, kidney hyperfiltration and proteinuria with minimal development of interstitial fibrosis. This is an animal model of early stage diabetic nephropathy rather than late stage diabetic nephropathy, as evidenced by the minimal development of interstitial fibrosis. Homozygous db / db (diabetic) are hyperglycemic at 8 weeks of age. Homozygous db / db (diabetic) and heterozygous db / +(non-diabetic) animals we...
example 2
CTGF Implicated in Early Stage Features of Progressive Vitreoretinal Disorders
[0117] The association between CTGF and ocular disease, including retinal disorders, has been previously established. (See, e.g., International Publication No. WO 03 / 049773.) Here, the relationship between ocular concentrations of CTGF and VEGF and the degree of neovascularization and fibrosis were examined to determine the correlation, if any, between CTGF and VEGF expression in vitreous. A correlation would be suggestive of CTGF involvement in both early stage and late stage aspects of ocular disorders. Undiluted vitreous samples (0.5 to 1 ml) were obtained at the start of a pars plana vitrectomy in patients with proliferative vitreoretinopathy (PVR), proliferative diabetic retinopathy (PDR), macular pucker, or macular hole. Samples of vitreous fluids were collected in sterile tubes, immediately frozen in dry ice, and stored at −80° C. until assayed for CTGF and VEGF.
[0118] Neovascularization associate...
example 3
Treatment of Late Stage Features of Diabetic Nephropathy
[0122] The effect of anti-CTGF therapy was examined in an animal model of late stage diabetic nephropathy. As has been previously described using this animal model, rats with diabetes mellitus exhibited high susceptibility to unilateral renal ischemia reperfusion, resulting in rapidly progressive nephropathy and end-stage renal failure, associated with development of fibrosis, atrophy of the kidney, and severely compromised glomerular filtration rate. (See, e.g., Melin et al. (1997) Kidney Int 52:985-991.) In this animal model of diabetes mellitus, ischemia severely impaired kidney function in diabetic rats. In this animal model, the renal effects on kidney function and pathology of hyperglycemia and ischemia are similar to those observed in human late stage diabetic nephropathy and end-stage renal disease (ESRD).
[0123] Diabetes mellitus was induced in male Sprague Dawley rats by a single i.v. dose of streptozotocin (STZ) (50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com