Methods for delivering DNA to muscle cells using recombinant adeno-associated virus virions vectors
a technology of adeno-associated virus and a delivery method, which is applied in the direction of recombinant dna-technology, genetic material delivery route, botany apparatus and processes, etc., can solve the problems of low expression level, ineffective long-term gene therapy, and decrease the life of the transduced cell, so as to achieve efficient delivery of genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of rAAV-LacZ in Terminally Differentiated Adult Rat Cardiomyocytes
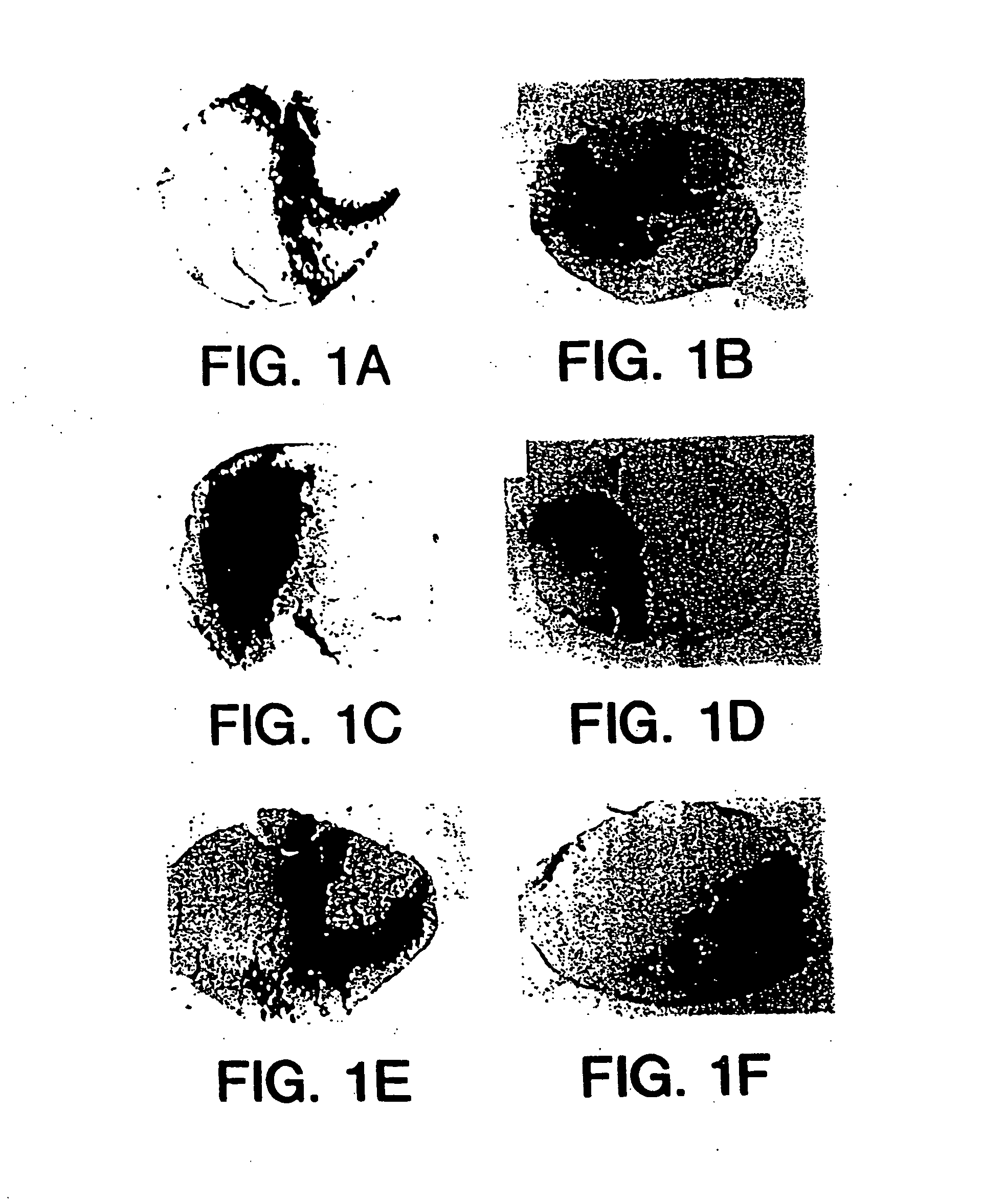
[0124] The ability of recombinant AAV virions to transduce terminally differentiated adult cardiomyocytes was established in vitro. Cardiomyocytes were harvested by coronary perfusion with collagenase of adult rat hearts (Fischer 344, Harlan Sprague Dawley, Indianapolis, Ind.). Cardiomyocytes were grown on laminin-coated glass coverslips and exposed to rAAV-LacZ virions for 4 hours. After 72 hours, the cells were stained for β-galactosidase activity. AAV expression was detected by blue staining of the binucleated cells. These studies demonstrated the ability of rAAV virions to transduce terminally differentiated cells. The transduction efficiency in vitro was 30% of adult cells at a multiplicity of infection (MOI) of 104 genomes per cell.
example 2
Stability of rAAV-LacZ Expression In Vivo
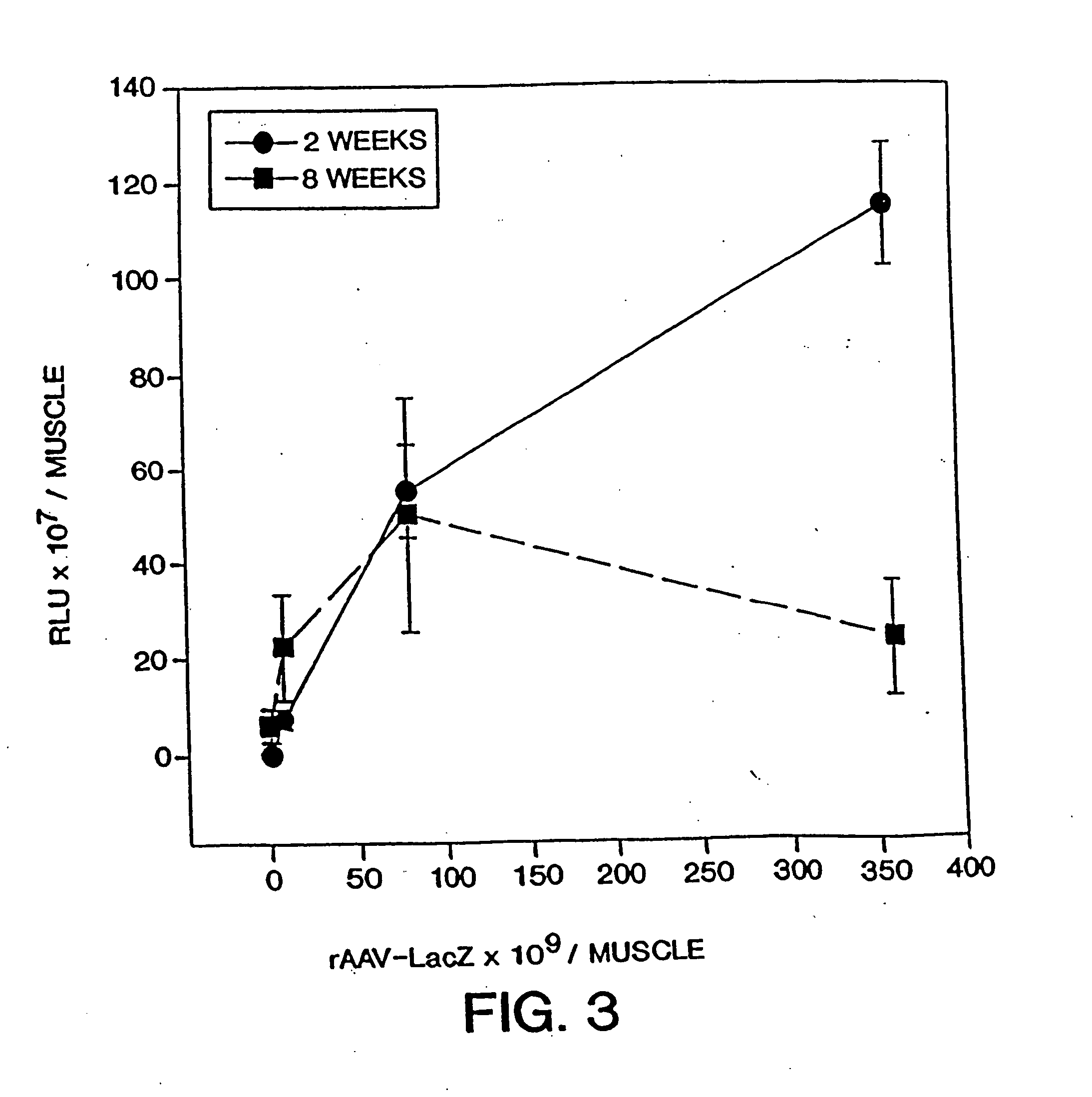
[0125] Adult Fischer rats were used to analyze expression of transgenes in vivo. Incremental doses of rAAV-LacZ virions were injected into the left ventricular apex of the heart using either a subxyphoid or lateral thoracotomy approach. More particularly, experimental animals were anesthetized with Metofane followed by a subxyphoid incision to expose the diaphragmatic surface of the heart. Apical cardiac injections were performed with a glass micropipette. Recombinant virion was diluted in normal saline and injected at a volume of 20-50 μL.
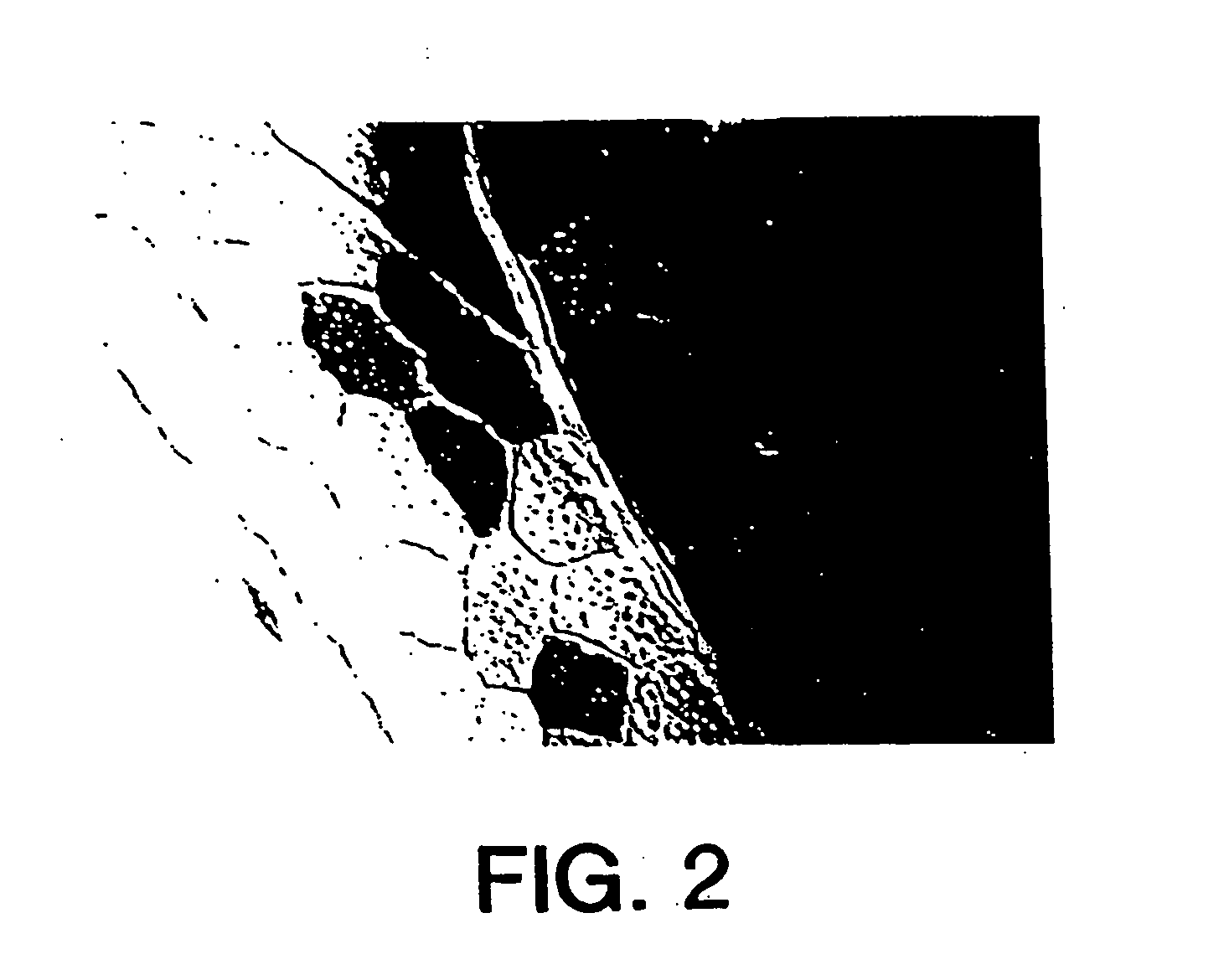
[0126] At varying times post-injection, hearts were harvested and examined for β-galactosidase production and for the presence of infiltrating mononuclear cells. For 5-Bromo-4-chloro-3-indolyl β-D-galactoside histochemical determination, frozen sections (6 μm) were fixed in 0.5% glutaraldehyde and stained for β-galactosidase activity as described (Sanes et al. (1986) “Use of Recombinant Retrovirus to Stud...
example 3
In Vivo Transduction of Murine Skeletal Muscle using rAAV-LacZ Virions
[0128] Recombinant AAV-LacZ virions were injected into muscle tissue of mice, and transduction assessed by β-gal activity. Particularly, in vivo transduction was performed by intramuscular (IM) injection of recombinant AAV virions into the skeletal muscle of healthy 6-8 week old Balb / c mice (Jackson Laboratories, Bar Harbor, Me., Simonsen Laboratories, Gilroy, Calif., or Harlan Laboratories) under either Metofane (Pitman-Moore, Mundelein, Ill.) or ketamine-xylazine anesthesia. The mid-portion of each tibialis anterior muscle was exposed via a 1 cm incision. Injections into the tibialis anterior were carried out using a micro-capillary tube attached to a Hamilton syringe to administer the following formulations at a depth of 2 mm: phosphate-buffered saline.(PBS) alone (negative control); or PBS containing either rAAV-LACZ virions or pW1909adhlacZ plasmids.
[0129] Tissue samples of tibialis anterior muscle, forelim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com