Methods for treating or predicting risk of a ventricular tachyarrhythmia event
a ventricular tachyarrhythmia and risk prediction technology, applied in the field of ventricular tachyarrhythmia risk prediction, can solve the problems of increased work and oxygen demand, less heart pump efficiency, increased blood flow, etc., and achieve the effect of slowing down the heartbeat more regularly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Soluble ST2 can be Used to Assess the Risk of a VTA in Subjects with Stable Heart Failure
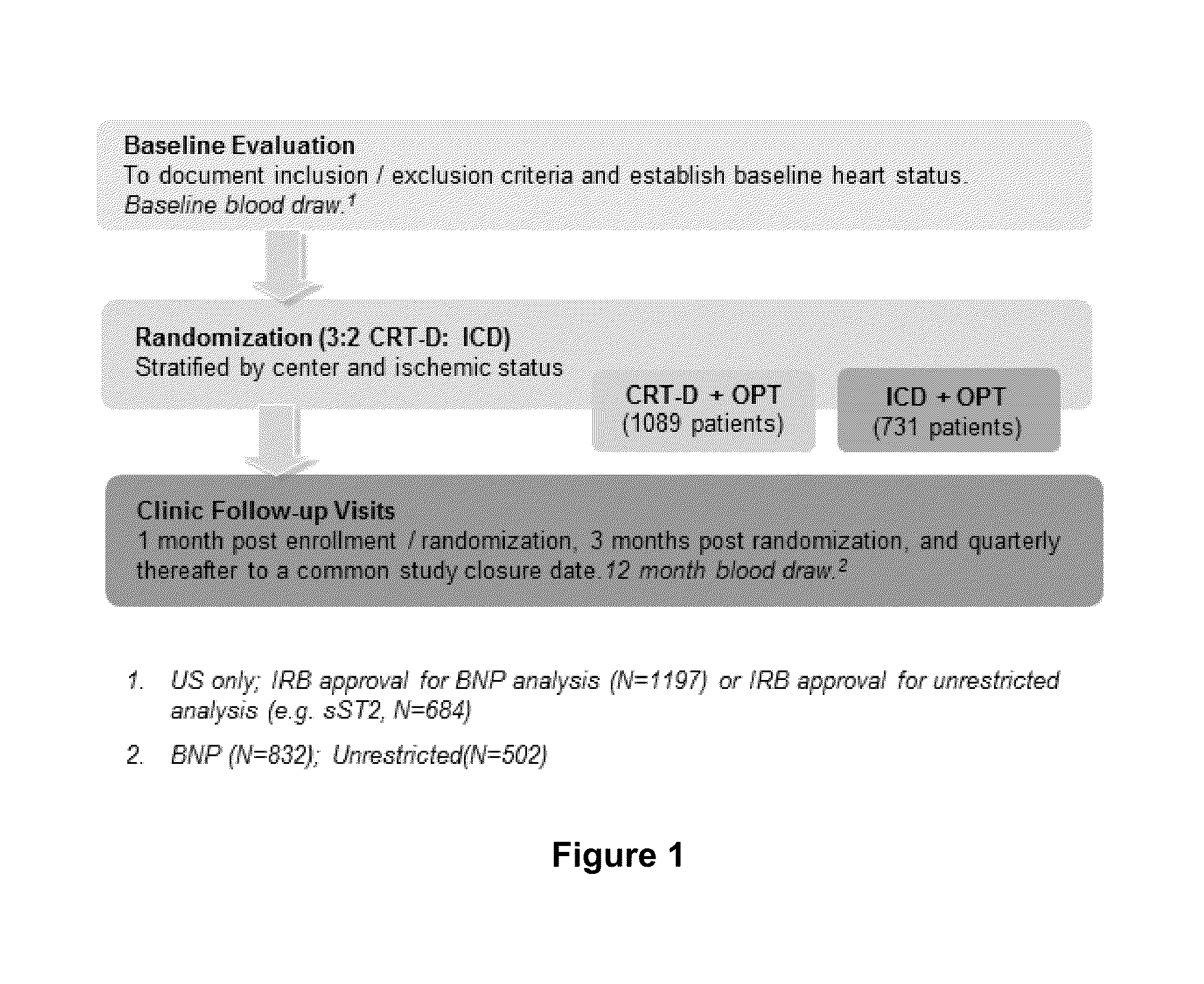
[0113]A set of experiments was performed to determine if soluble ST2 (ST2) is useful for predicting the occurrence of VTA events in stable class I / II heart failure patients who were receiving treatment with an ICD or CRT-D (subjects enrolled in the MADIT-CRT trial). A schematic of the MADIT-CRT study design is shown in FIG. 1. The MADIT-CRT is the largest randomized NYHA Class I / II ICD / CRT-D trial to date. A total of 1820 patients were enrolled in this study at 110 centers in 14 countries. The average follow-up time for subjects participating in this study was 34.3 months. Commercially available devices were used in these studies (Boston Scientific, Natick, Mass.).
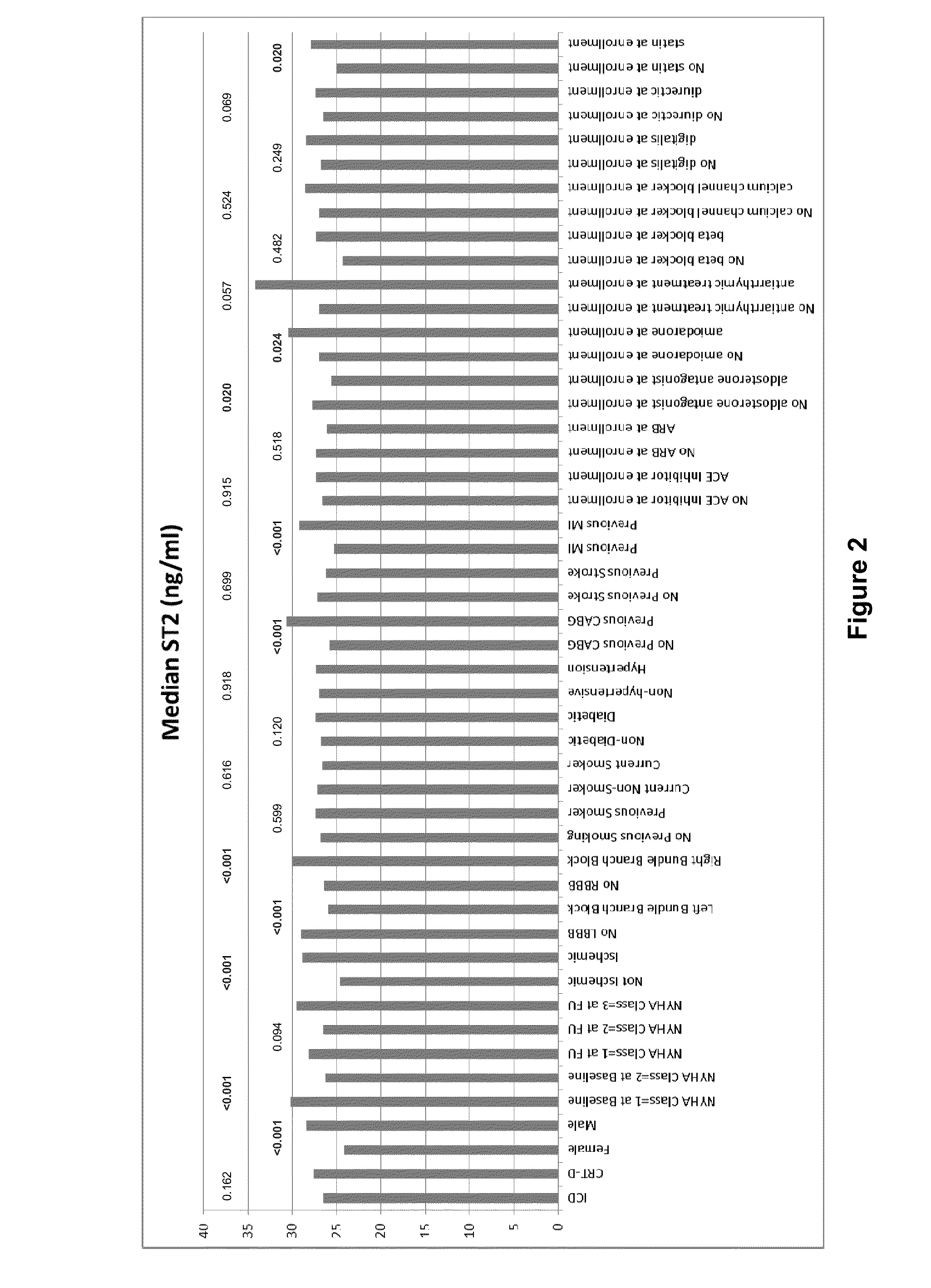
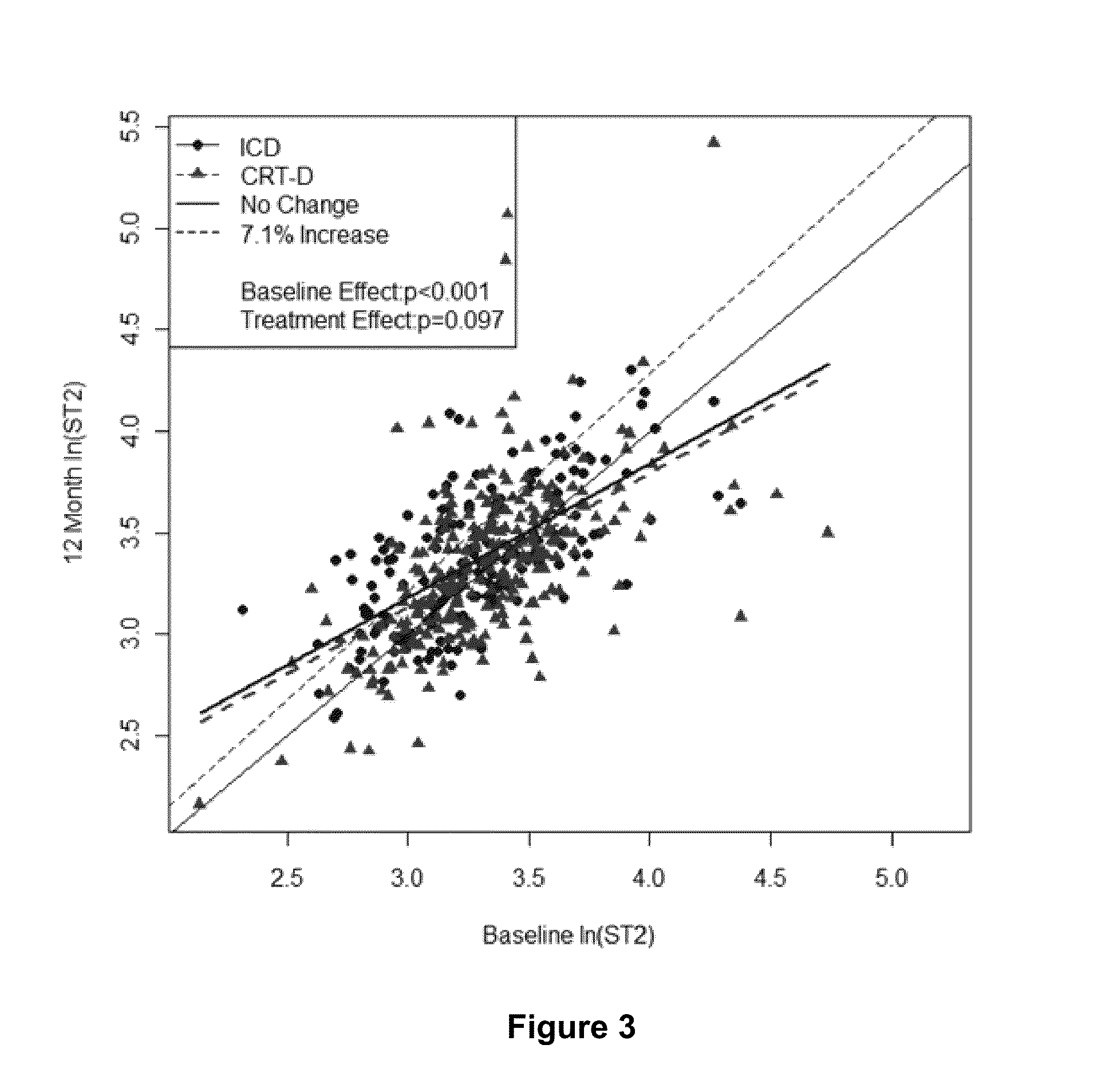
[0114]In these experiments, soluble ST2 and BNP levels were measured at baseline and at 1 year in patients participating in this MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy) sub-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com