Methods and compositions for preparing and purifying noribogaine
a technology of alkaloids and compositions, applied in the field of methods and compositions for purifying the nonaddictive alkaloids of noribogaine, can solve the problems that the one-step method for preparing noribogaine from ibogaine via demethylation does not provide the requisite assurance that ibogaine will be consistently removed, and achieve the effect of reducing the probability of contamination by ibogain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Purification of Noribogaine from Ibogaine
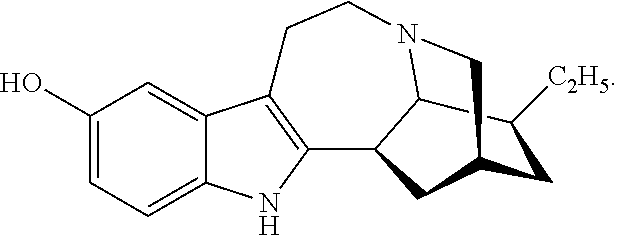
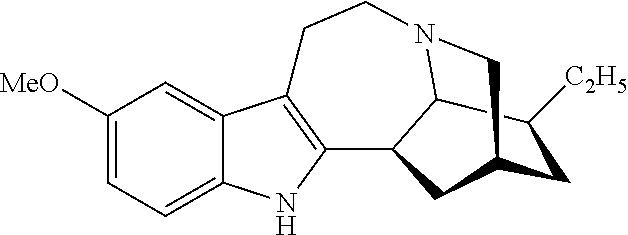
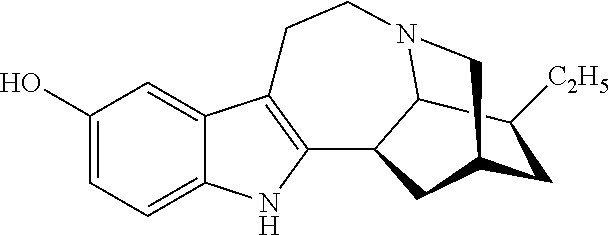
[0055]Example 1 illustrates one method for the synthesis and purification of noribogaine from ibogaine which method follows Scheme 4 below:
[0056]Specifically, in Scheme 4, ibogaine is contacted with a stoichiometric excess of benzyl chloroformate in an inert solvent such as methylene chloride. The reaction mixture further contains at least a stoichiometric equivalent of diisopropylethylamine relative to ibogaine so as to scavenge the acid generated during the reaction. The reaction is maintained at room temperature under an inert atmosphere until the reaction is substantially complete as evidenced by, for example, thin layer chromatograpy. At which time, an O-demethylation reagent (e.g. boron tribromide, aluminum trichloride, or lithium diphenylphosphine), or preferably a stoichiometric excess thereof, is added to the reaction mixture which is then maintained under conditions (e.g. room temperature) wherein the methoxy group of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com