Novel Antibody And Use In Diagnosis And Therapy Of Arthropathies
a technology of arthropathies and antibodies, applied in the field of new antibody and use in diagnosis and therapy of arthropathies, can solve the problems of increasing the risk of serious infections and malignancies, inconsistent effectiveness, and increasing the number of patients that fail anti-tnf therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
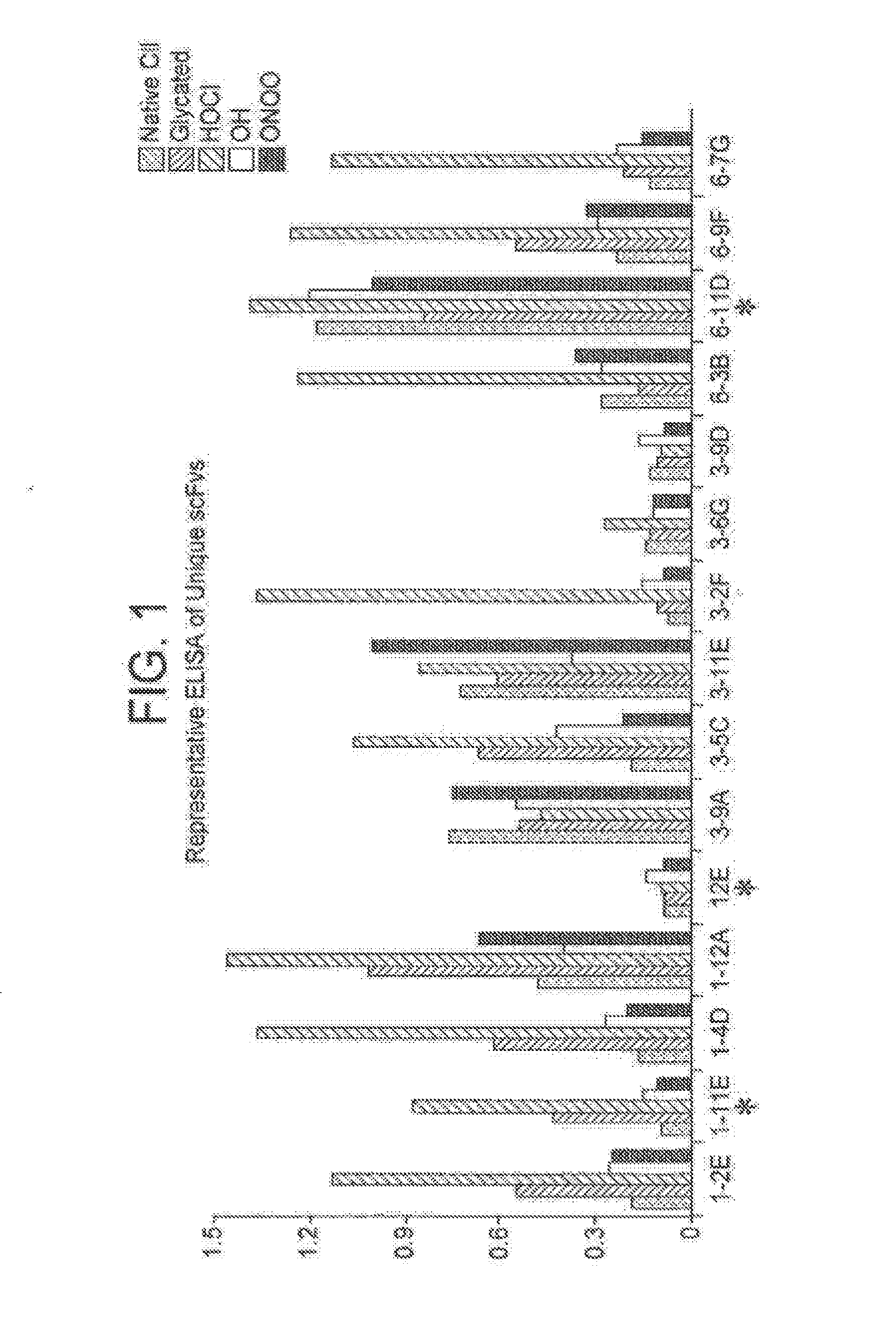
Selection of Anti-Modified CII scFv from Phase-Display Library
[0087]Phage display antibody technology (Winter G et al, Annu. Rev. Immunol. 12: 433-455, 1994) was used to raise a single chain fragment variable (scFv) that binds only to CII that has been post-translationally modified by free radicals.
[0088]A human semi-synthetic scFv library constructed from a single human framework for VH (DP-47 and JH4) and VL (DPK9 and JK1) was employed, in which diversity was incorporated in CDR3 and CDR2 (de Wildt R. M et al, Nat. Biotechnol. 18(9): 989-994, 2000). To select for phage binding to modified CII and not to native non-modified CII, subtractive selection was performed using native non-modified CII for subtraction. HOCl modified CII was used as a target for panning as binding to HOCl modified CII was strongest in RA sera (Nissim A, 2005). Glycated CII was used in parallel. Briefly, immunotubes (Nunc-Immuno Tubes, Maxi-Sorp, Nunc, Denmark) were coated with 10 μg / ml CII in phosphate-buffe...
example 3
Screening and Sequencing of Modified CII-Specific Phage Clones
[0089]Screening for positive anti-modified CII phage clones was first performed by enzyme-linked immunosorbent assay (ELISA), as previously described (Harrison J. L, 1996). Microtiter plate (Nunc, Paisley, UK) wells were coated with 10 μg / ml native or modified CII and incubated with 100 μl phage suspension for 90 minutes. In addition, native and modified BSA were used as negative control. After removal of the supernatants, the amount of bound phage was determined using peroxidase-labeled anti-M13 antibodies (GE Healthcare: Ltd, Little Chalfont, Buckinghamshire) and developed by using 100 mM 3,3′5,5′ tetramethylbenzidine (TMB) as substrate. The reaction was monitored in an ELISA reader at 450 nm with a reference wavelength of 650 nm using GENios plate reader (TECAN, Theale Court, Reading UK) and Magellan software (TECAN, Theale Court, Reading UK)
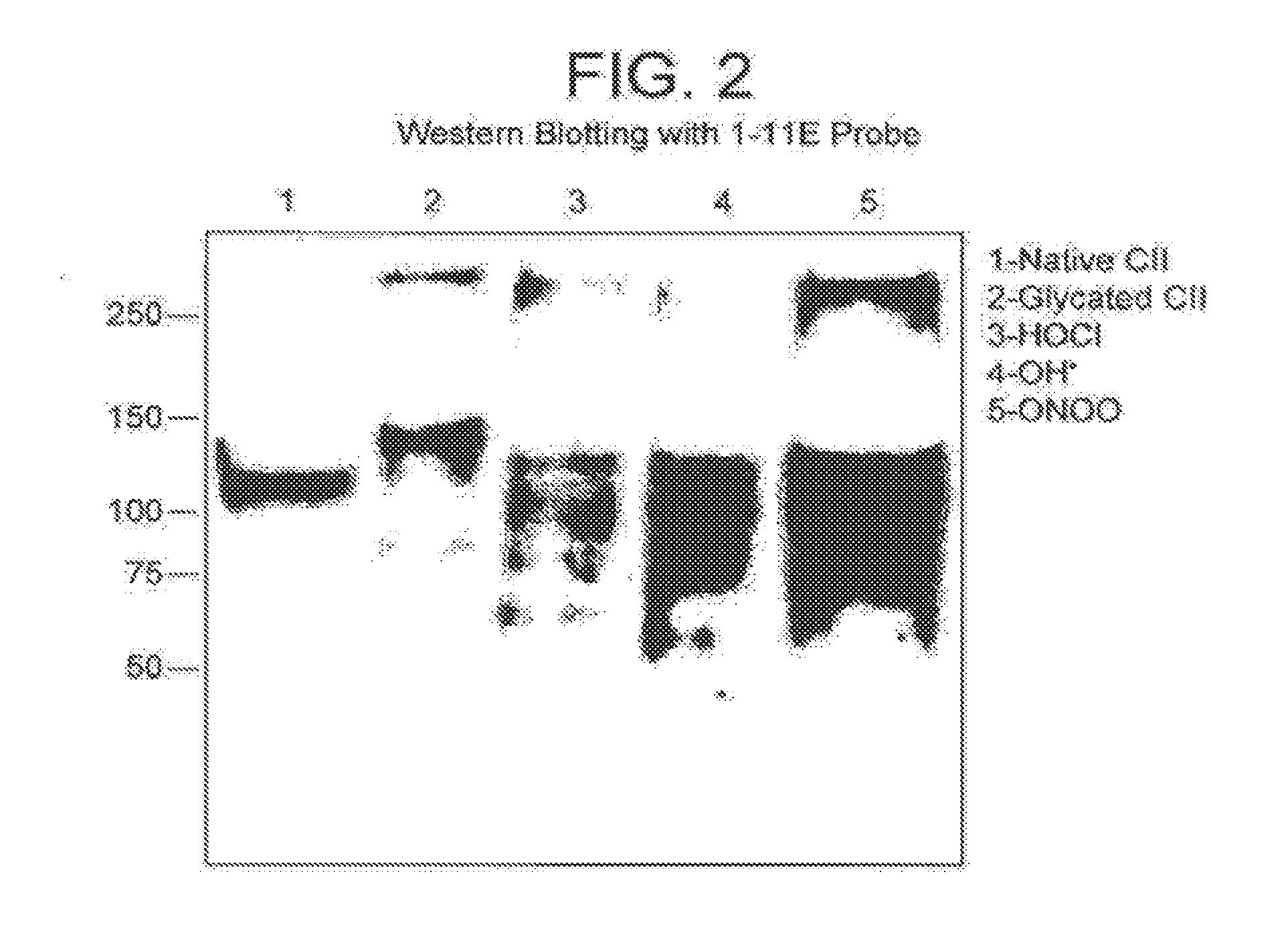
[0090]The entire scFv DNA fragment of each modified CII bound phage clone was ...
example 4
Production and Purification of Anti-Modified CII-scFv
[0091]The reactive phage clones obtained from E. coli TG-1 bacteria were used to infect E. coli HB2151 non-suppressor bacterial strain to obtain soluble scFv. After overnight induction with 1 mM IPTG at 30° C., the antibody fragments, derived from the VH3 family, were harvested from the supernatant and periplasmic space as described (Harrison J. L, 1996) and purified on a protein A affinity column (GE Healthcare Ltd, Little Chalfont, Buckinghamshire). Binding of purified scFv to modified CII was first analyzed by ELISA as above except that mouse anti-myc tag antibody (Santa Cruz Biotechnology, INC, Wembley, UK) followed by anti-mouse-HRP conjugate (Sigma, Dorset, UK) were used to probe bound scFv.
Anti-Modified CII scFv Raised by Phage Display Human Antibody Library
[0092]After three rounds of subtractive selection 82 phage clones specific to either glycated CII or HOCl modified CII were selected out of which 42 clones had unique se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com