Widespread gene delivery to motor neurons using peripheral injection of aav vectors
a technology of aav and aav, which is applied in the direction of muscular disorders, immunological disorders, drug compositions, etc., can solve the problems of failure of classical pharmacology, difficult alternative supply of mn with recombinant proteins injected directly into the cns parenchyma, and no treatment for these diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ular Injection of mSEAP-Expressing AAV Vectors in the Neonatal Mouse
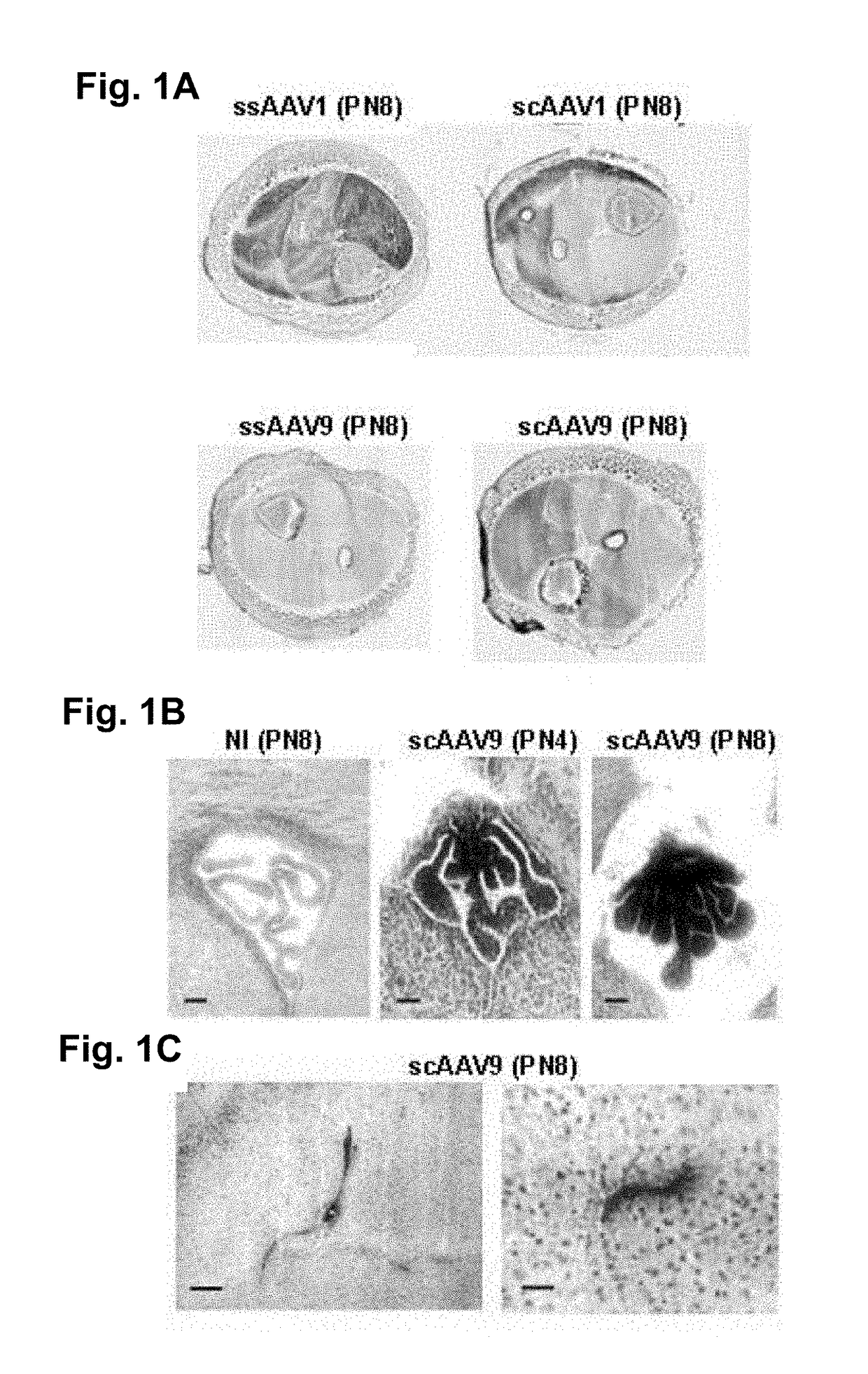
[0090]We first evaluated the potential of serotype 1 and 9 ss- or scAAV vectors to transduce the CNS cells after i.m. injection. The ssAAV1, ssAAV9, scAAV1 or scAAV9 encoding the murine secreted alkaline phosphatase (mSEAP) under the cytomegalovirus (CMV) promoter were injected into both triceps and gastrocnemius muscles in one day old C57Bl6 mice (8.10+9 to 2.10+10 viral genome per mouse, 3 mice per group). The injected muscle, brain and spinal cord tissues were removed 1, 3 or 7 days post injection and analysed for mSEAP expression using histochemistry.
[0091]The mSEAP expression was detected in the injected muscles 3 and 7 days after injection of each AAV serotype, except with ssAAV9, the expressing level dramatically increasing with time (FIG. 1a). In the CNS, transgene expression was detected only after i.m. injection of scAAV9. Interestingly, the mSEAP expression was detected in the epithelial cells of the chor...
example 2
toneal Injection of mSEAP-Expressing AAV Vectors in the Neonatal Mouse
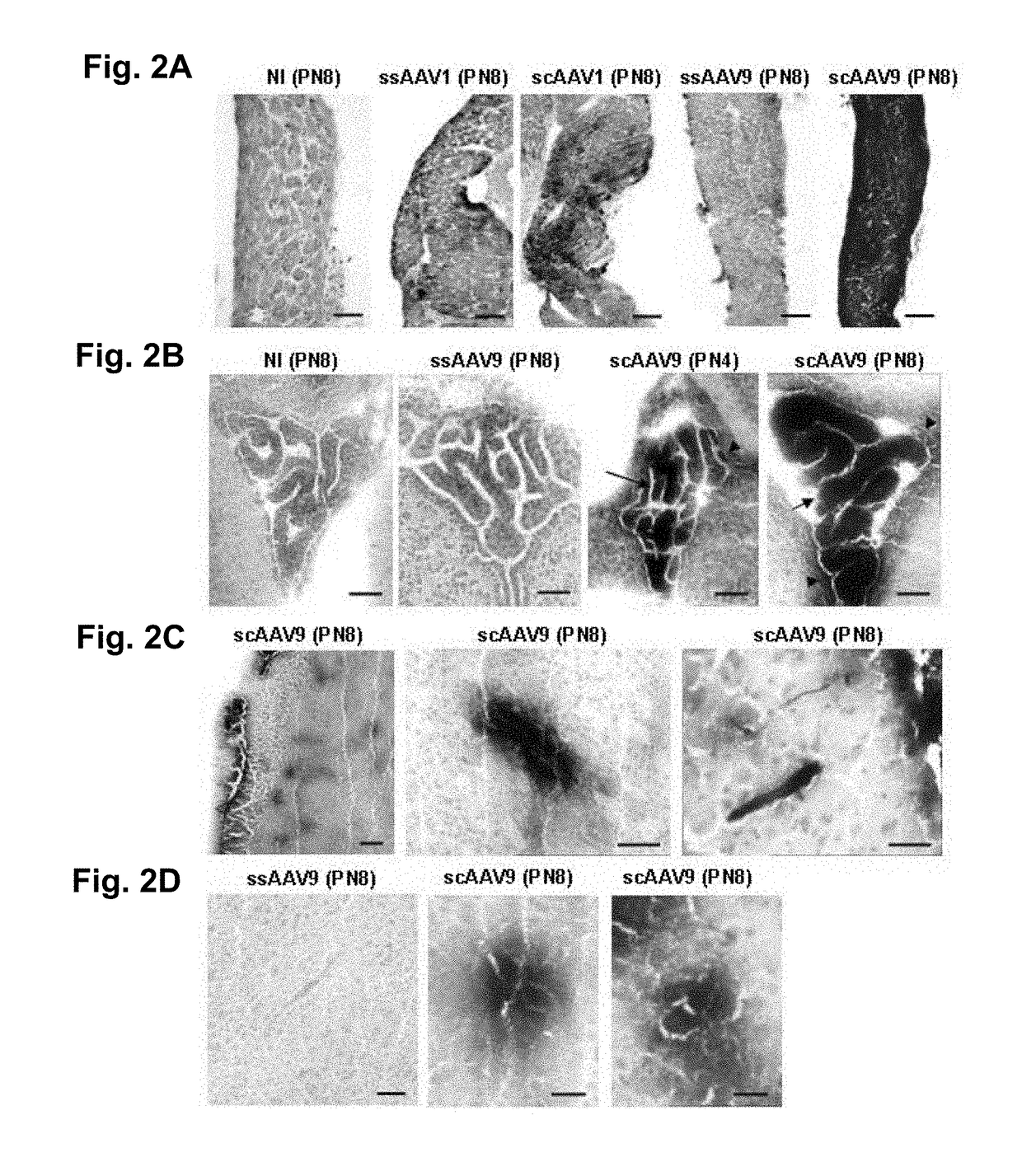
[0092]We then analysed whether i.p. administration of ssAAV1, ssAAV9, scAAV1 and scAAV9 in one day old C57Bl6 mice (100 μl, 3·10+10 to 1·10+11 viral genome per mouse) could mediate transgene expression in the CNS at 1, 3 or 7 days post injection.
[0093]A low level of mSEAP expression was detected in the diaphragm muscle fibres of mice injected with ssAAV1 by 3 days post-injection, which was similar to that observed with scAAV1 (FIG. 2a), and with both vectors at 7 days after injection. (FIG. 2a). SsAAV9 transduce a few muscle fibres only at 21 days post injection, whereas an intense mSEAP staining was found in the diaphragm when using scAAV9 from 3 days post-injection (FIG. 2a). This high level of transduction was also observed in other muscles such as the triceps brachii or gastrocnemius muscle (data not shown).
[0094]The epithelial cells of the choroids plexus and the ependyma appeared clearly labelled after injec...
example 3
Expression in the CNS after i.m. Or i.p. Injection of scAAV9-GFP in the Neonatal Mouse
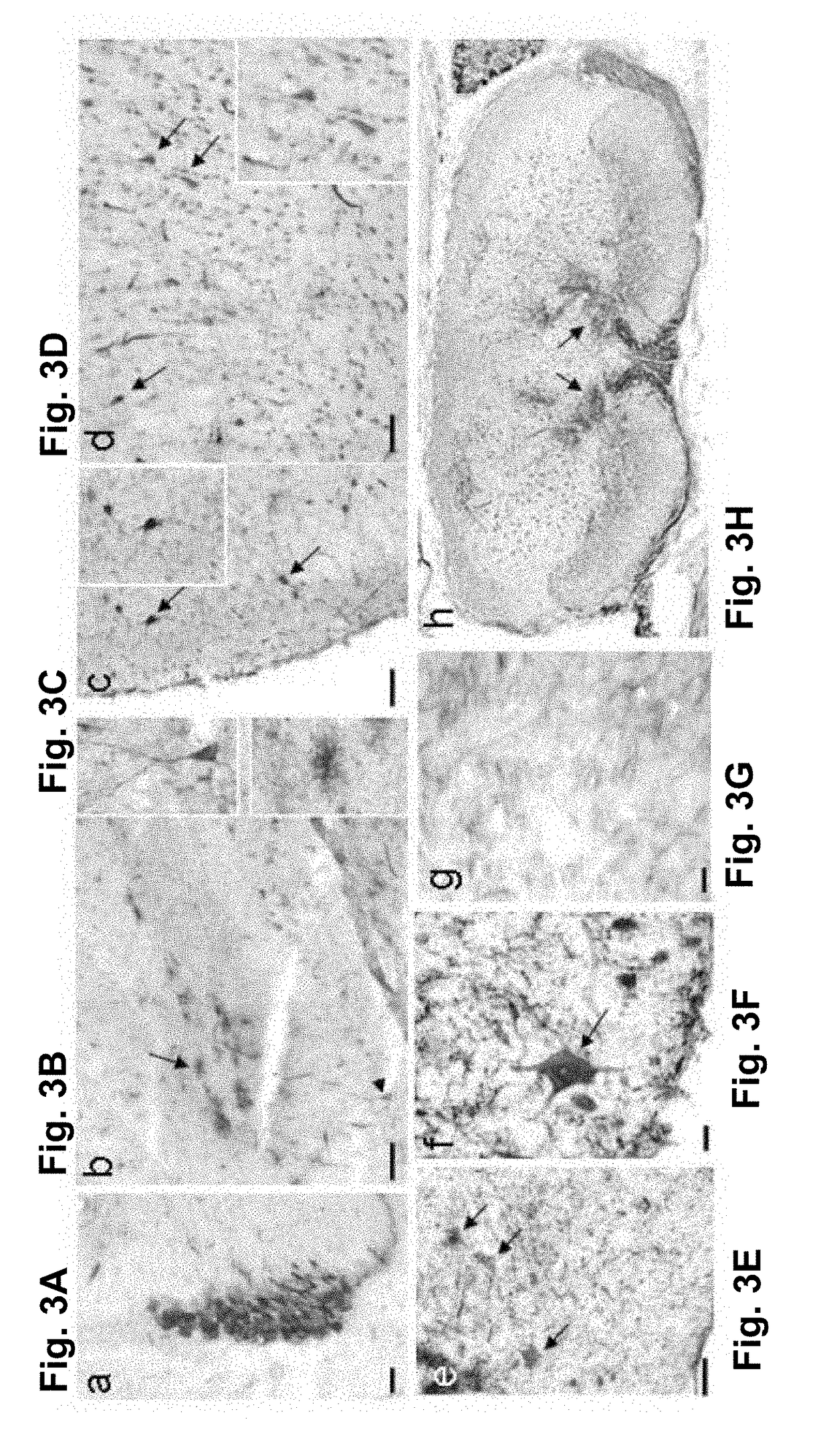
[0095]Since mSEAP is a secreted protein, the transgene expression observed in the CNS cells after peripheral AAV injection could result from protein transcytosis rather than from AAV cell transduction. We thus verified whether similar results could be obtained when using a non-secreted protein.
[0096]A recombinant scAAV9 vector expressing the “green fluorescent protein” (GFP) was injected in neonatal mice either intraperitoneally (3.10+10 vg per mouse, 100 μl) or intramuscularly (8.10+9 vg in 20 μl per mouse, 5 μl per muscle). Seven days later, and similarly to that observed with scAAV9-mSEAP, the GFP expression was observed in the choroids plexus and ependyma cells located in the brain ventricles (FIG. 3a and FIG. 4a). Furthermore, we found in this case many GFP-positive neural cells in several brain regions located, in particular, close to the ventricles. Cell bodies and fibres in the hippocampus ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com