Hemagglutinin-specific antibodies and uses thereof
a technology of hemagglutinin and specific antibodies, applied in the field of hemagglutinin-specific antibodies, fragments thereof, can solve the problems of inability to meet the needs of patients, so as to speed up or improve the accuracy of quantification methods, and optimise the bioprocess used to generate vaccines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production and Purification of Antigen
[0139]A fusion peptide was prepared to use for immunization of mice. The fusion protein comprised a conserved peptide sequence from the N-terminal region of HA2 (GLFGAIAGFIEGGW; SEQ ID NO:26), functional groups on the peptide sequence, and keyhole limpet hemocyanin (KLH).
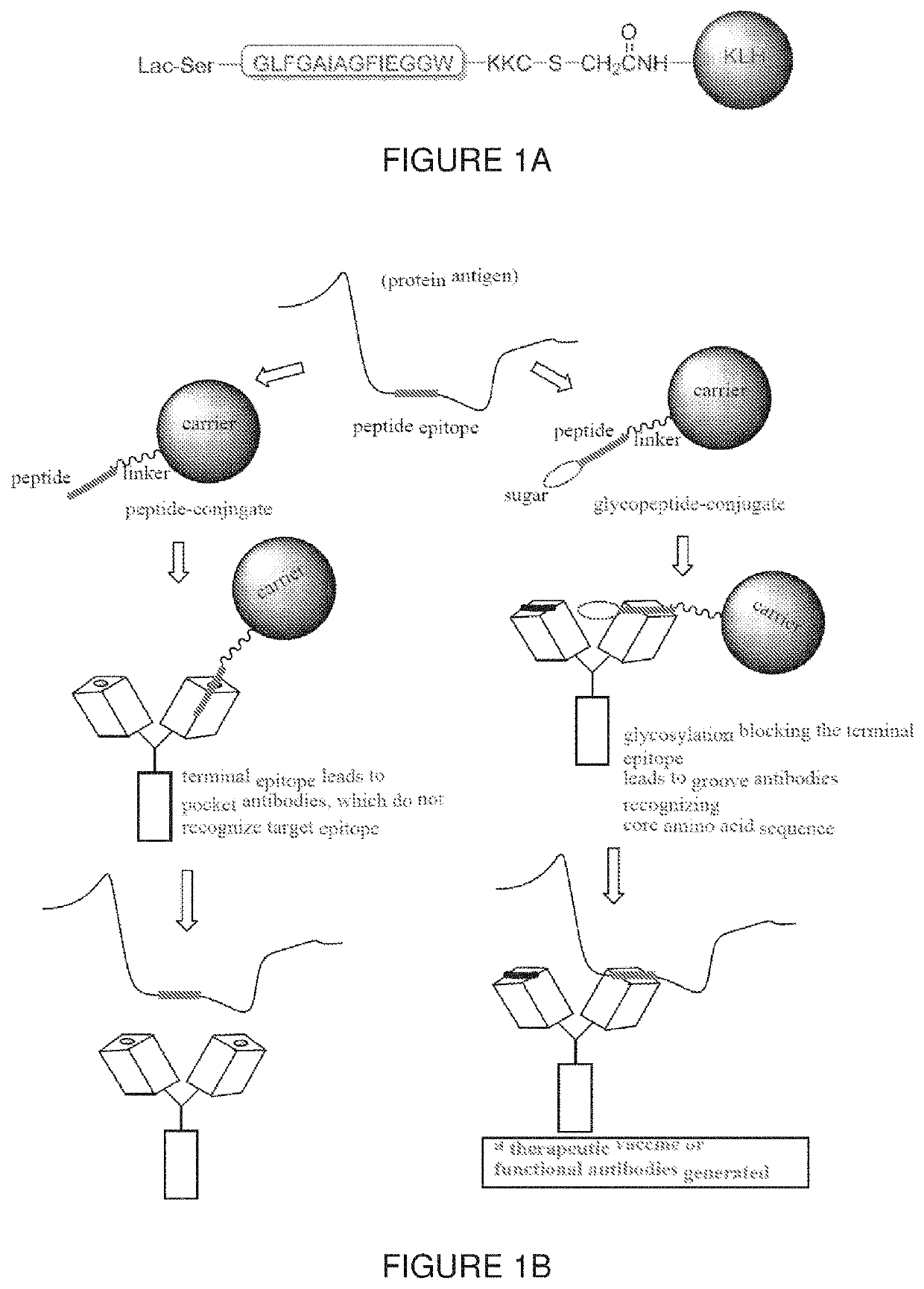
[0140]Conjugate structure. The peptide conjugate shown in FIG. 1A, was designed by using a conserved peptide sequence at the N-terminus of HA2 that was previously identified: GLFGAIAGFIEGGW (SEQ ID NO:26). The peptide epitope was functionalized with lactose and conjugated to Keyhole Limpet Hemocyanin (KLH) to obtain a conjugate of formula (I):
[0141]The KLH portion of the conjugate also comprised multiple epitopes conjugated to its surface.
[0142]Peptide conjugate synthesis. The peptide conjugate was prepared according to FIG. 1B via a thio-ether bond between terminal Cys of the (glyco)peptide antigen and bromoacetyl KLH.
[0143]Bromoacetylation of KLH. Typically, 20 mg of KLH (Sigm...
example 2
Generation of Anti-Influenza Antibodies
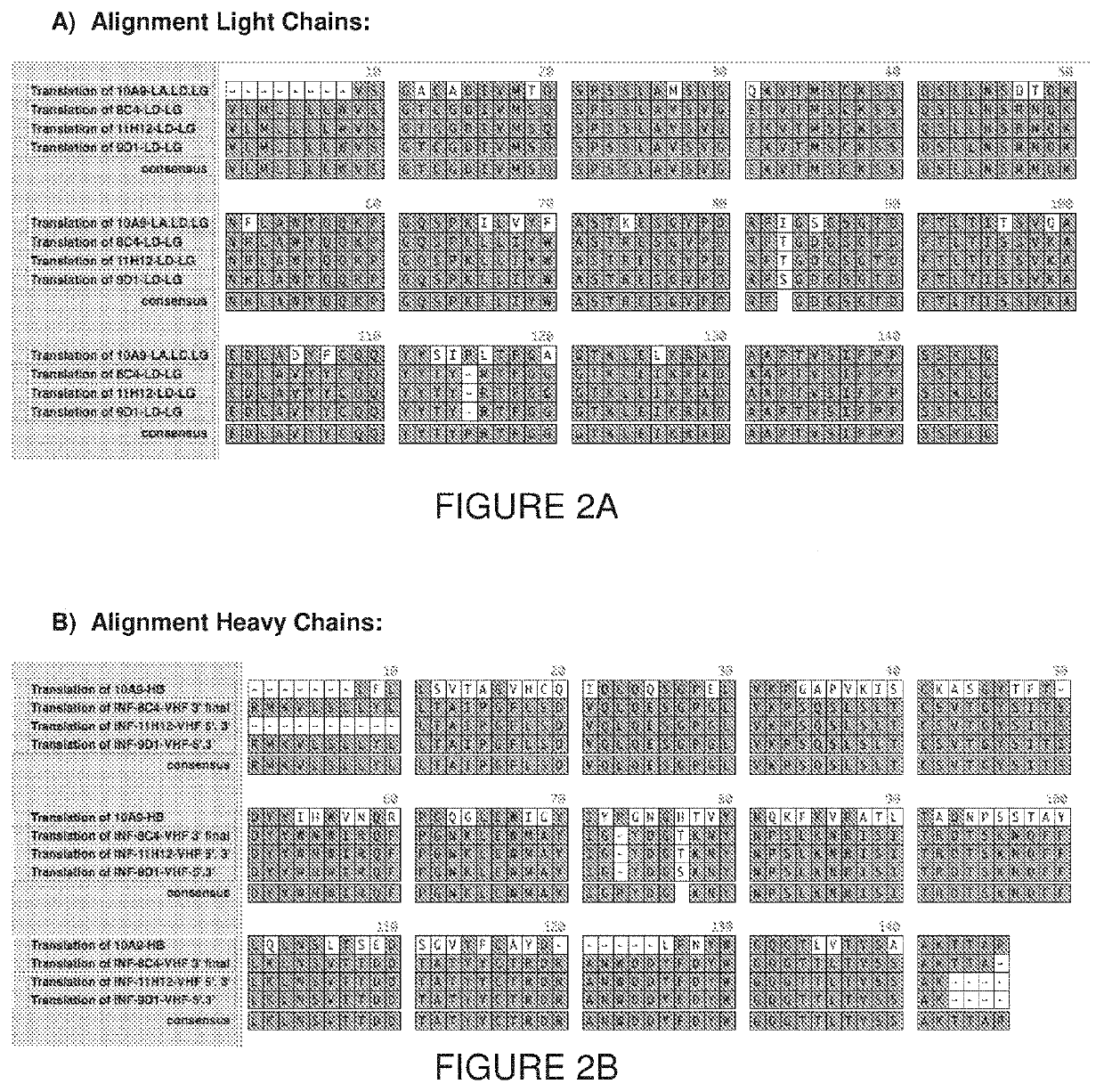
[0146]To produce antibodies that target the influenza virus, mice were immunized with the peptide conjugate obtained in Example 1. Hybridomas (monoclonal antibodies) were also prepared and evaluated by ELISA.
[0147]Immunizations. 6-week old A / J mice were bled (pre-immune serum) and immunized i.p. and s.c. with 100 μg of antigen (Example 1) in Titermax adjuvant. Three weeks later, a second injection of 100 μg of antigen in Titermax adjuvant was performed and mice were bled 7-10 days later. The serum titer was measured by ELISA. Two months later, a final i.p. booster injection using 100 μg of antigen was performed 4 days prior to fusion experiment.
[0148]Fusion of the harvested spleen cells. All manipulations were carried out under sterile conditions. Spleen cells were harvested from immunized mice in IMDM (Hy-Clone) and fused to NSO myeloma cell line using PEG fusion protocol. To this end, spleen cells and myeloma cells were washed in IMDM, counte...
example 3
Biophysical Characterization of the Anti-HA mAb
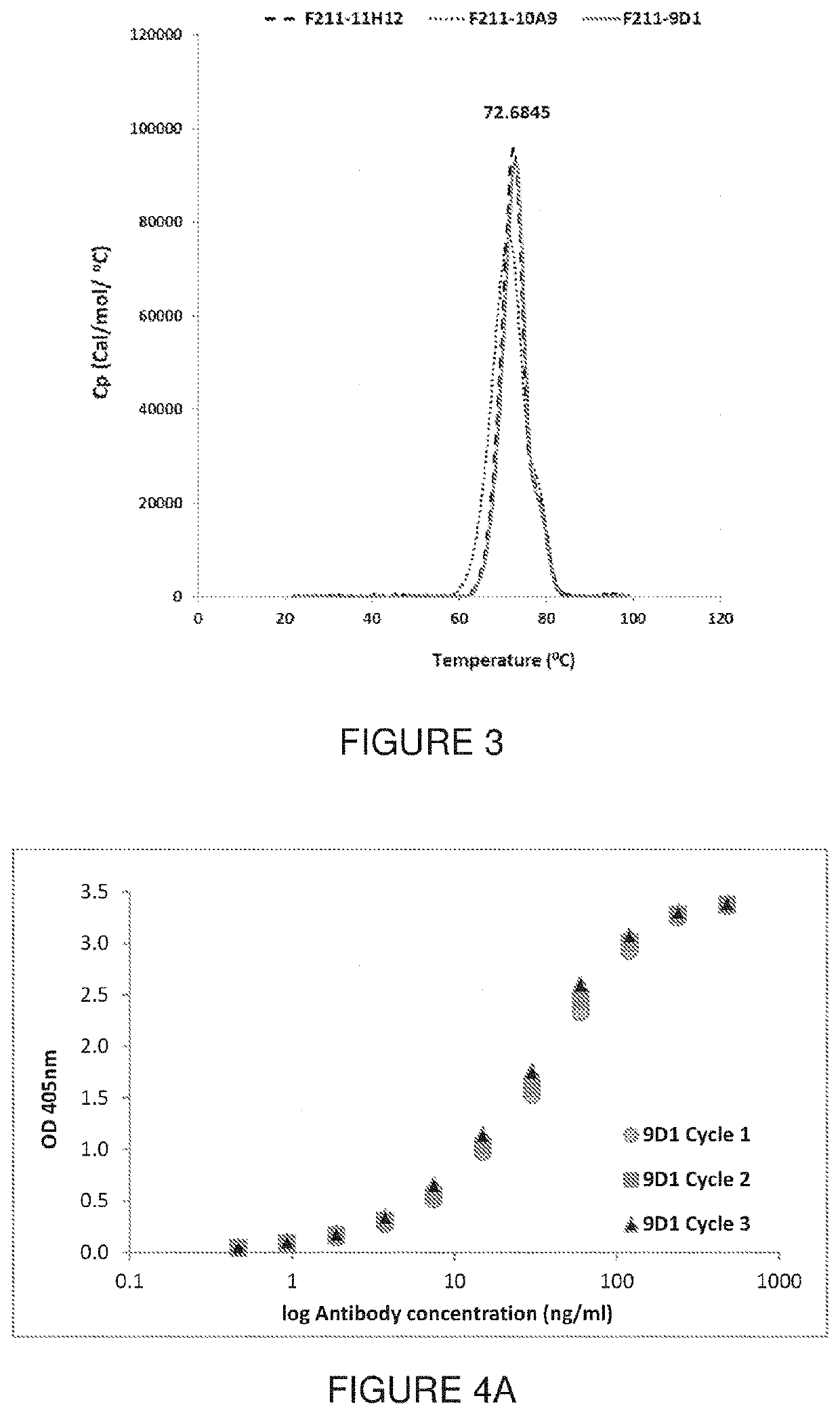
[0154]The anti-HA mAb of Example 2 were characterized using Differential Scanning calorimetry (DSC) and surface plasmon resonance (SPR). MAb resistance to freeze-thaw cycles were also evaluated.
[0155]Thermal stability of the mAb measured by DSC: The mAb were analysed by DSC to determine at which temperature unfolding of the mAb is induced. Each purified mAb was diluted to 0.4 mg / mL in PBS, and a total of 400 uL was used for DSC analysis with a VP-Capillary DSC (Malvern Inc.). At the start of each DSC run, 5 buffer blank injections are performed to stabilize the baseline and a buffer injection precede each mAb injection for referencing. Each sample was scanned from 20 to 100° C. at a 60° C. / hr rate, with low feedback, 8 sec filter, 5 min preTstat, and 70 psi nitrogen pressure. The mAb thermograms were referenced and analyzed using Origin 7 software. The melting points of the mAb were found to be between 71.28 and 72.90° C. as reported in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap