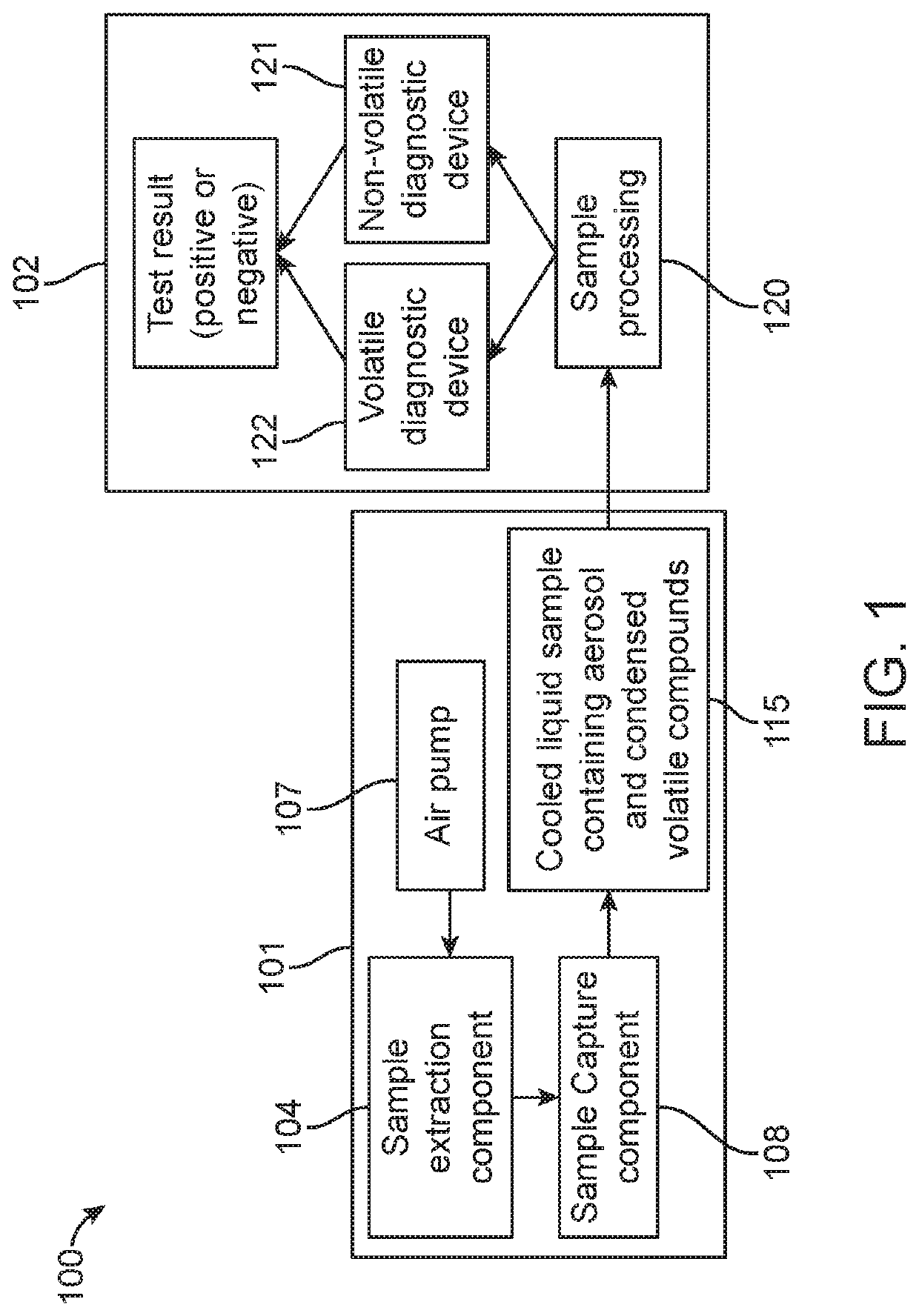
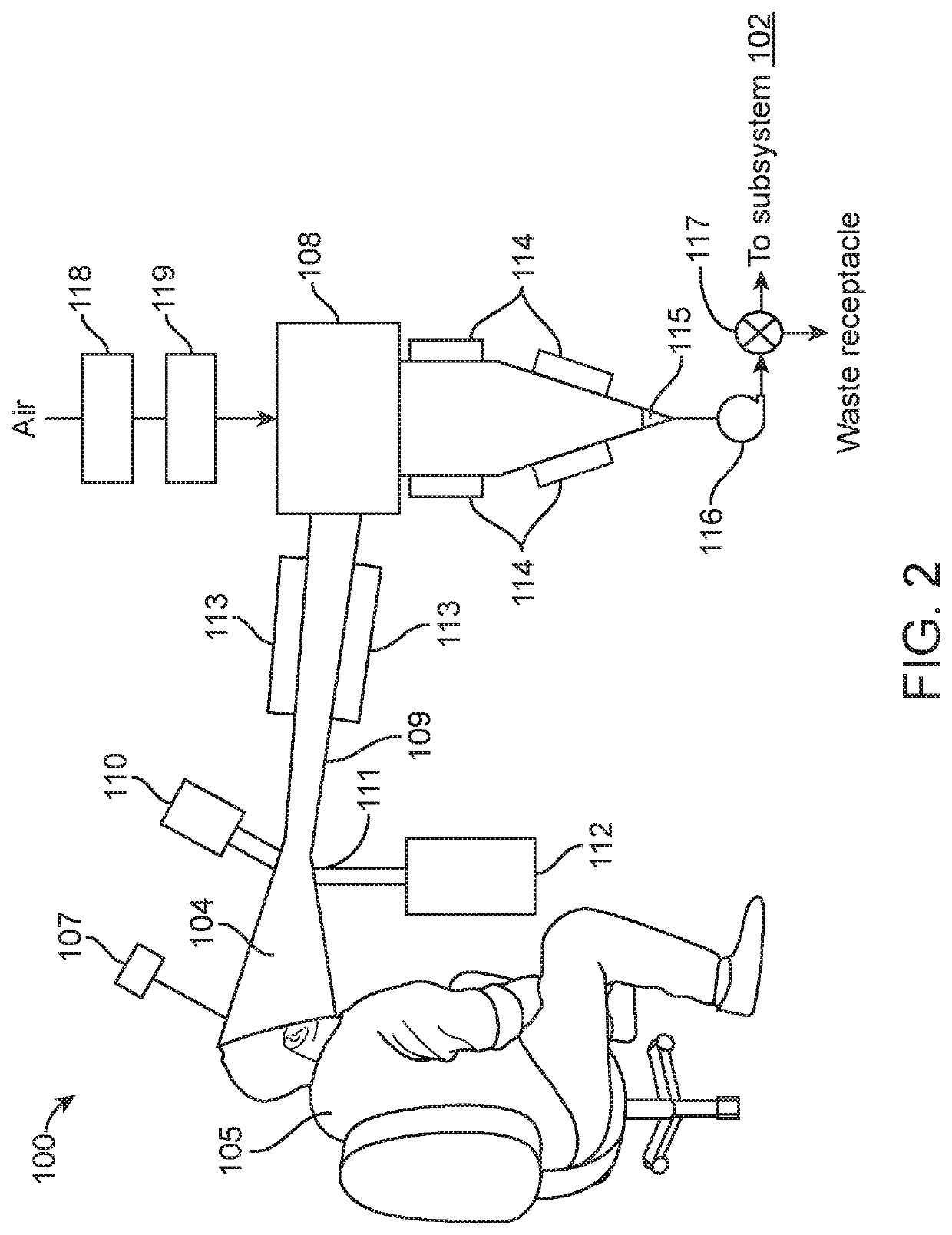
The rate of decline in incidence remains inadequate at a reported 1.5% per annum and it is unlikely that treatment alone will significantly reduce the burden of disease.
The physical process of TB transmission remains poorly understood and the application of new technologies to elucidate key events in infectious aerosol production, release, and inhalation, has been slow.
Two major difficulties plaguing investigation are the purportedly low concentrations of naturally produced Mtb particles, and the complication of environmental and patient derived bacterial and fungal contamination of airborne samples.
Therefore, interruption of transmission would likely have a rapid, measurable impact on TB incidence.
The need for sputum as a diagnostic sample is a limiting factor due to the challenges of collecting it from patients and to its complex composition.
The viscosity of the material restricts test sensitivity, increases sample-to-sample heterogeneity, and increases costs and labor associated with testing.
Moreover, sputum production (which requires coughing) is an occupational hazard for healthcare workers.
First, only about 50% of patients can provide a good sputum sample.
For example, children under about age of eight often are not able to produce a sample upon request, usually because they have not developed an ability to “cough up” sputum from deep in their throat.
The elderly and ill may not have the strength to cough up sputum.
Thus, a diagnostic method based on sputum analysis may not provide a diagnosis in as many as 50% of the patients who provide a sputum sample.
Sputum is also not useful as a diagnostic sample if it is collected one to two days after a person has been treated with antibiotics because the sample is no longer representative of the disease state deep in the lungs, and within several days after treatment begins, the number of live Mtb in the sputum is significantly reduced.
Blood is highly invasive and entails the higher cost of handling blood samples that are often HIV positive since, in some parts of the world, many TB patents also have HIV co-infections.
Further, a patient with an active TB infection may not have many TB cells circulating in their blood.
Urine-based diagnostics have also been proposed, but these tests look for biomarkers of the disease other than living TB bacilli, and none not been validated for widespread clinical use.
Characterized by uncertain and variable degrees of dilution, EBC may not provide precise assessment of individual solute concentrations within native airway lining fluid.
The diagnostic systems and methods such as sputum analysis and blood analysis are either not automated and autonomously operated, or not rapid.
Many have expensive assays that are consumed for each analysis, and thus, do not have general utility for active case finding, particularly in developing and under-developed countries.
First, the breath aerosol sample is collected into discrete samples that are several milliliters in volume, and thus, considerable effort is needed to concentrate the sample.
Further, the diagnostic device is not coupled to or integrated with the sample collector and is not amenable for use as an ACF tool.
As a result, if a sample is found to be negative for influenza it may be due to a false negative resulting from inadequate sample collection.
It is well known that there are large variabilities among humans with respect to the volume of aerosolized lung fluid produced during various breathing maneuvers.
It may be integrated with an EBA sample collection method to perform ACF of TB and other respiratory diseases, but the sample collection times would be too long to be practical.
However, the GeneXpert Ultra assay has a relatively high cost per test and takes approximately an hour to complete the assay and provide a result.
In general, PCR-based diagnostics are unsuitable for TB screening for ACF applications due to both the extended time needed for sampling and analysis, and the relatively high cost per test.
If not, the test is considered to be too slow and not acceptable for achieving short patient wait-times. In the developing world, and especially in countries with a history of TB prevalence, the GeneXpert may be used to provide diagnosis in about one hour.
As previously described, this assay is expensive to implement on a “cost per test” basis, and therefore it is not yet widely deployed.
Culturing of impacted samples took 30-60 days, and therefore this approach is not amenable to automation.
A challenging aspect of EBA as a clinical sample is the relatively small sample of volume of exhaled particulates that can be collected from breath.
Further, a significant fraction of the mass collected is water.
Exhaled breath analytical tools have not been commercialized because methods and devices to efficiently collect and concentrate the trace amounts of analyte present in exhaled breath are lacking.
Furthermore, there is no standard or methodology to assess how much exhaled breath is sufficient for a particular diagnosis.
 Login to View More
Login to View More