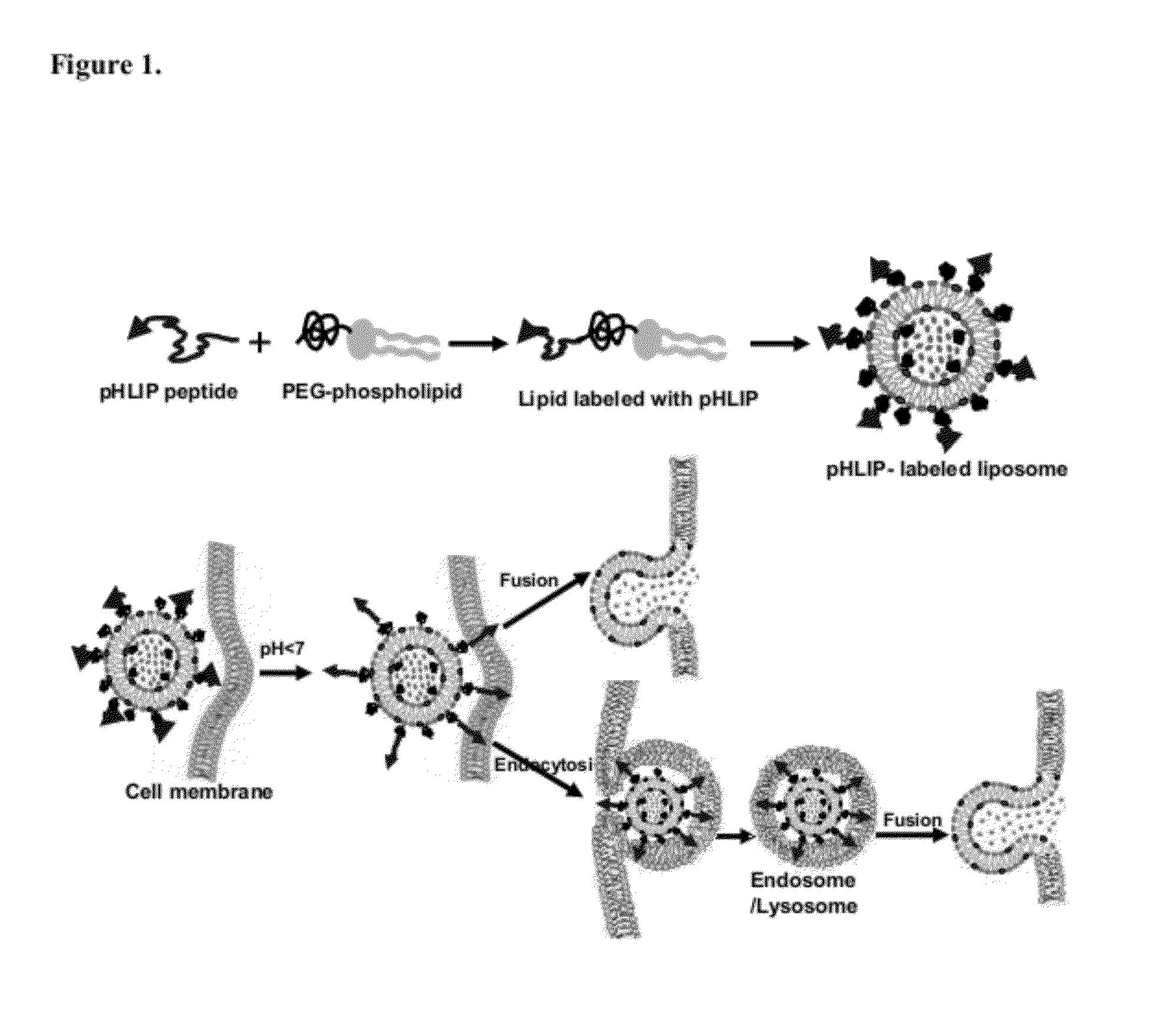
Liposome compositions and methods of use thereof
a technology of liposomes and compositions, applied in the direction of drug compositions, biocide, genetic material ingredients, etc., can solve the problems of difficult to rely on any single tumor biomarker, difficult to identify and treat cancer cells while sparing non-cancerous cells, and heterogeneity of human cancers, so as to reduce the binding/insertion of phlips and the phlip labeling or targeting of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
pHLIP-Mediated Delivery of Liposomes
[0089]The following regents and methods were used to generate the data described herein.
Lipids
DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
[0090]
DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
[0091]
DSPE-PEG(2000) Maleimide
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt)
[0092]
DSPE-PEG(2000)
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]
[0093]
Fluorescein DHPE
N-(fluorescein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt
[0094]
Rhod PE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl
[0095]
R18 (Octadecyl Rhodamine B Chloride)
[0096]
Conjugation of pHLIP with Lipids
[0097]pHLIP containing Cys on the N-terminus and DSPE-PEG(2000) Maleimide lipids. Standard maleimide chemistry reaction was applied in methanol. (Reaction of thiol with maleimide.)
[0098]
where
R1—DSPE-PEG(2000) Maleimide (MW 2941)
R2...
example 2
pHLIP Enhances Uptake of Liposomes by Cells
[0113]pHLIP (pH-Low-Insertion-Peptide) insertion into membrane occurs as a result of protonation of Asp / Glu residues due to a decrease of pH. Protonation enhances peptide hydrophobicity and increases its affinity for a lipid bilayer, which triggers peptide insertion and formation of transmembrane helix. Since many pathological states are associated with the development of elevated level of extracellular acidity (or low extracellular pH), pHLIP-liposomes are ideally suited for selective delivery of diagnostic and therapeutic agents to the cancer cells. Attachment of cargo molecules to the N-terminus (FIG. 14).
[0114]One approach to deliver gold material (or cytotoxic compounds) to tumor is to use liposomes. In contrast to fusogenic liposomes developed before for delivery, which can fuse with cellular membrane only in the absence of PEG coating, pHLIP mediates fusion between lipid bilayer of plasma membrane or membrane of endosome / lysozome and...
example 3
pHLIP Promotes Distortion of Plasma and Endosome Membranes, the Release of R18-Labeled FA into the Cytoplasm, and Targeting of Mitochondria
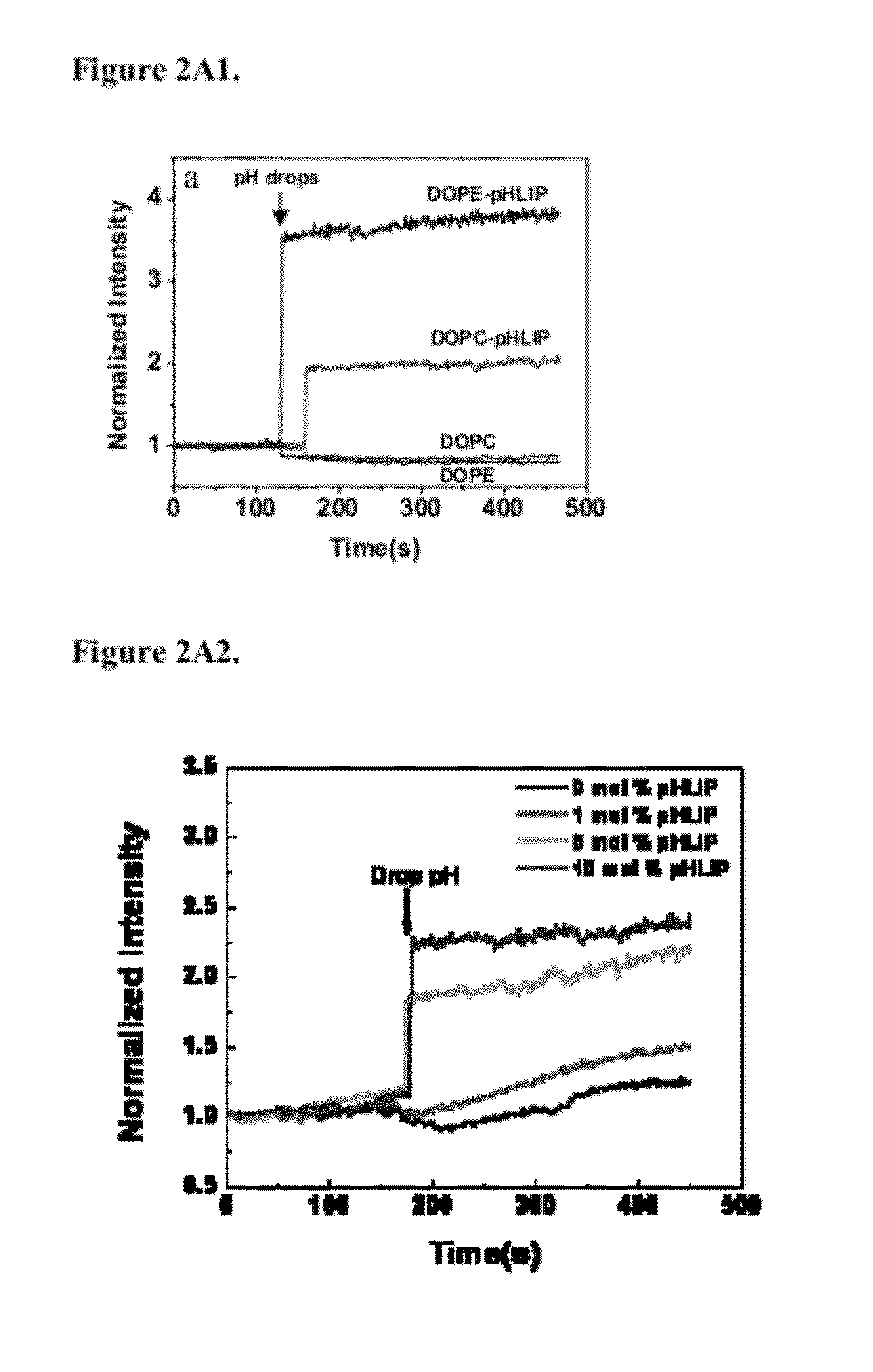
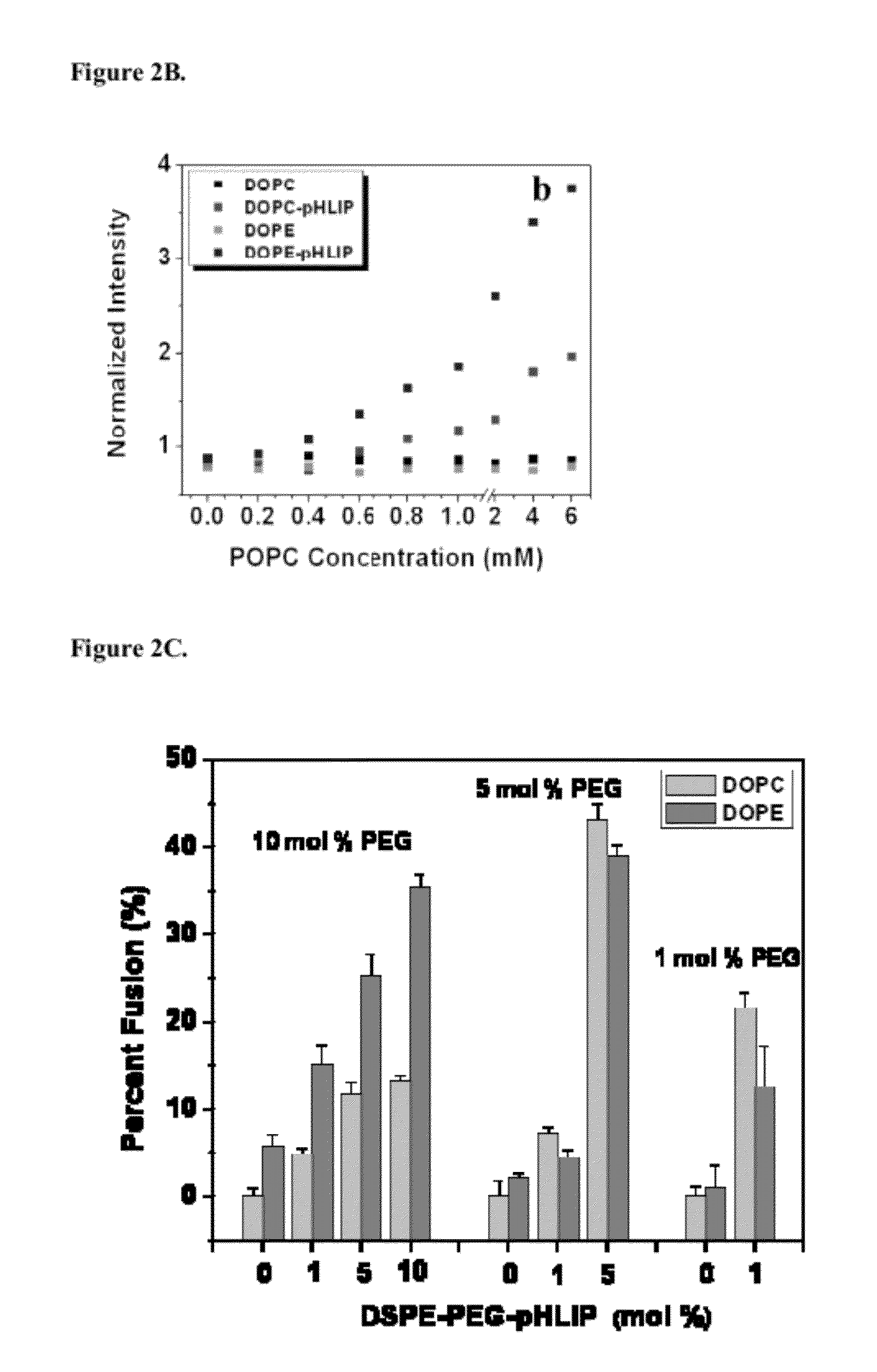
[0117]After cellometer counting, cells were reseeded in collagen-coated cell dishes for microscopy imaging. Cellular localization of fluorescent fatty acids (R18) incorporated into liposomes containing PEG polymers and pHLIP or no pHLIP on the surface (non-fusogenic DOPC lipids were used in study). In case of liposomes, fluorescent signal was mostly localized in endosomes, while pHLIP promotes distortion of plasma and endosome membranes and release of R18-labeled FA into cytoplasm and targeting of mitochondria. FIG. 9 shows the localization of Rho-labeled liposome in cells.
[0118]FIG. 11 shows images of Fluorescein-labeled liposomes fused with a cellular membrane. FIG. 11(a) phase contrast; (b) FITC. Co-localization of FITC-liposome (c) and plasma membrane staining of red-fluorescent Alexa Fluor594 wheat germ agglutinin (d). The data demonstrate t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com