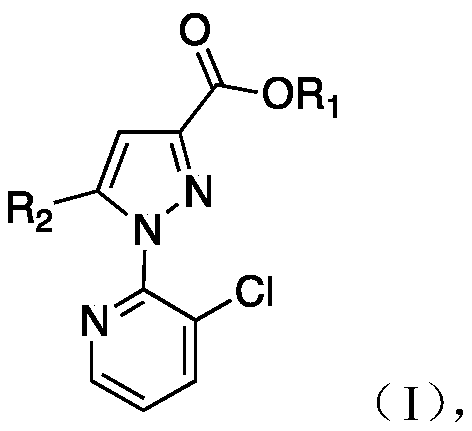
Application of compound containing 1-(3-chloro-2-pyridyl)-1H-parazole active fragment in producing fungicide
An active fragment, pyridyl-based technology, applied in the field of pesticides, to achieve the effect of unique structure, broad-spectrum and high-efficiency bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
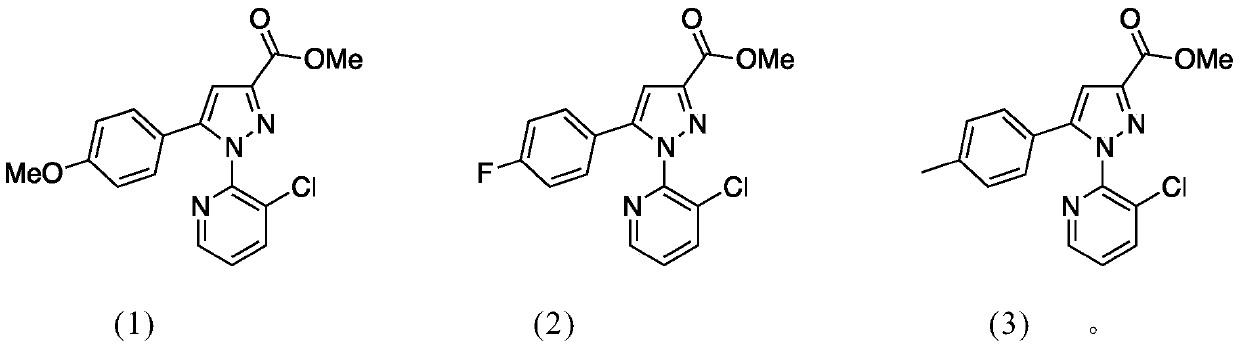
[0028] The preparation of embodiment 1 compound (1)
[0029] a. Add metal sodium 4.5g (200mmol) to 70ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add p-methoxyacetophenone 15g (100mmol) and diethyl oxalate 17.5g (120mmol), After the dropwise addition, stir at room temperature for 4 hours, add 2mol / L hydrochloric acid to adjust the pH value to 2-3, filter the resulting solid substance 21g of methyl p-methoxyphenyl 2,4-dicarbonylbutyrate, and the yield is 90 %;
[0030] b. Add 20g (100mmol) p-methoxyphenyl 2,4-dicarbonylbutanoic acid methyl ester and 18g (100mmol) 3-chloropyridine-2-hydrazine hydrochloride in the round-bottomed flask filled with methanol, stir After refluxing for 24 hours, solids were precipitated, filtered, and recrystallized from ethanol to obtain 28 g of the target compound, with a yield of 80%.
Embodiment 2
[0031] The preparation of embodiment 2 compound (2)
[0032] a. Add 4.5g (200mmol) of sodium metal to 70ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add 14g (100mmol) of p-fluoroacetophenone and 17.5g (120mmol) of diethyl oxalate, dropwise After completion, stir at room temperature for 4 hours, add 2 mol / L hydrochloric acid to adjust the pH value to 2-3, filter the resulting solid substance 20 g of methyl p-fluorophenyl 2,4-dicarbonyl butyrate, and the yield is 87%;
[0033] b. Add 19.6g (100mmol) methyl p-fluorophenyl 2,4-dicarbonyl butyrate and 18g (100mmol) 3-chloropyridine-2-hydrazine hydrochloride to a round-bottomed flask filled with methanol, and stir to reflux After 24 hours, solids were precipitated, filtered, and recrystallized from ethanol to obtain 25.9 g of the target compound, with a yield of 75%.
Embodiment 3
[0034] The preparation of embodiment 3 compound (3)
[0035] a. Add 4.5g (200mmol) of sodium metal to 70ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add 13.5g (100mmol) of p-methylacetophenone and 17.5g (120mmol) of diethyl oxalate, After the dropwise addition, stir at room temperature for 4 hours, add 2 mol / L hydrochloric acid to adjust the pH value to 2-3, filter the resulting solid substance p-methylphenyl 2,4-dicarbonylbutanoic acid methyl ester 22.6g, yield 92 %;
[0036] b. Add 19.2g (100mmol) p-methylphenyl 2,4-dicarbonylbutanoic acid methyl ester and 18g (100mmol) of 3-chloropyridine-2-hydrazine hydrochloride to the round bottom flask filled with methanol and stir After reflux for 24 hours, a solid precipitated, filtered, and recrystallized from ethanol to obtain 31.4 g of the target compound, with a yield of 87%
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap