A kind of 1,5-diaryl-3-carboxylate pyrazole compound, preparation method and use
A technology of methyl pyrazoles and ester pyrazoles, applied in the field of pesticides, can solve the problem that pyrazole-3-formyl compounds are not widely used, and achieve broad-spectrum high-efficiency bactericidal activity, good control effect, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of compound (1)
[0047] (1) The synthetic process of compound 1a:
[0048]
[0049] Add metal sodium (0.46g, 0.02mol) in batches to a flask containing 100ml of anhydrous methanol, stir, and add diethyl oxalate (2.92g, 0.02mol) and 4'-fluorobenzene Ethanone (1.38 g, 0.01 mol) was mixed, and the mixture was stirred and reacted at room temperature for 2-3 hours. After the reaction was completed, it was acidified to neutral, and a large amount of solids precipitated out. The product 1a could be obtained after filtration and draining. It could be used in the next reaction to prepare compound (1) without further purification. The purity of the obtained compound 1a was 90%, and the yield was 90%. The detection results of compound 1a are as follows:
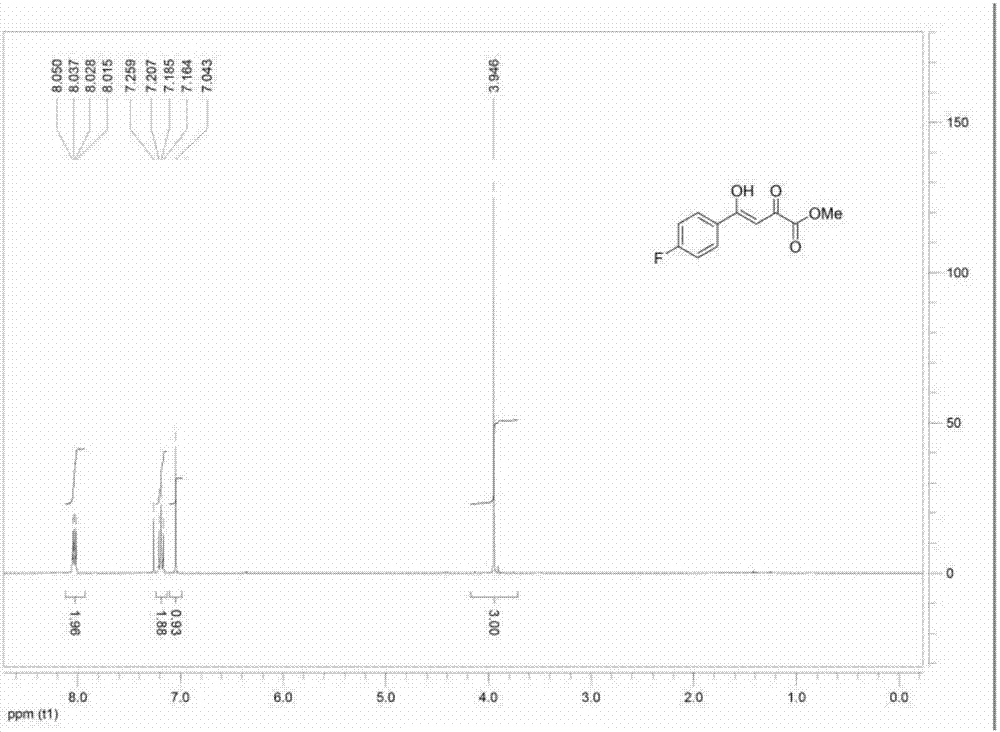
[0050] 1 HNMRδ:8.05(q,2H,J 1 =8.4Hz,J2 =5.2Hz, Ar-H); 7.19(t, 2H, J=
[0051] 8.4Hz, Ar-H); 7.04(s,1H,CH); 3.94(s,3H); ESI-MS: m / z 247.1[M+Na] + ,Such as figure 1 shown.
[0052] (2) The sy...
Embodiment 2
[0055] Embodiment 2: the synthesis of compound (2)
[0056] (1) Synthetic process of compound 2a
[0057]
[0058] The synthesis process of compound 2a is the same as that of compound 1a, except that the raw materials used are 100 ml of anhydrous methanol, 0.02 mol of sodium metal, 0.02 mol of diethyl oxalate, and 0.01 mol of p-methylacetophenone. The purity of the obtained compound 2a was 90%, and the yield was 95%. The detection results of compound 2a are as follows:
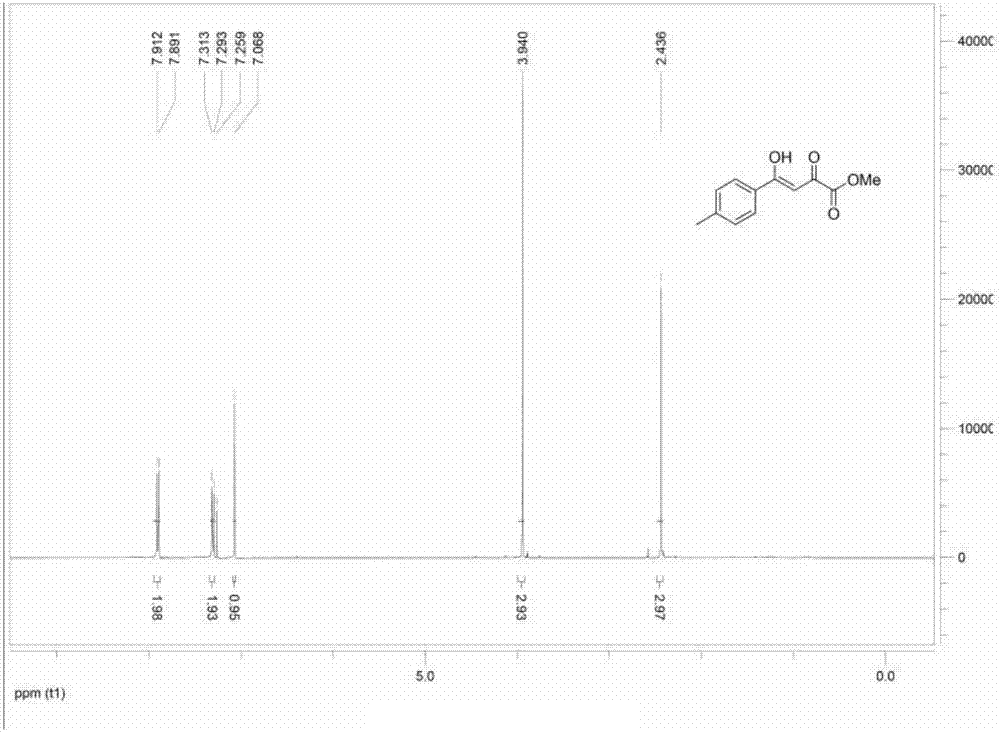
[0059] 1 HNMRδ: 7.91 (d, 2H, J = 8.4Hz, Ar-H); 7.31 (d, 2H, J = 8.4Hz, Ar-H); 7.06 (s, 1H, CH); 3.94 (s, 3H, - OCH3); 2.37(s,3H,-CH3); ESI-MS: m / z 243.1[M+Na] + .Such as image 3 shown.
[0060] (2) Synthetic process of compound (2)
[0061]
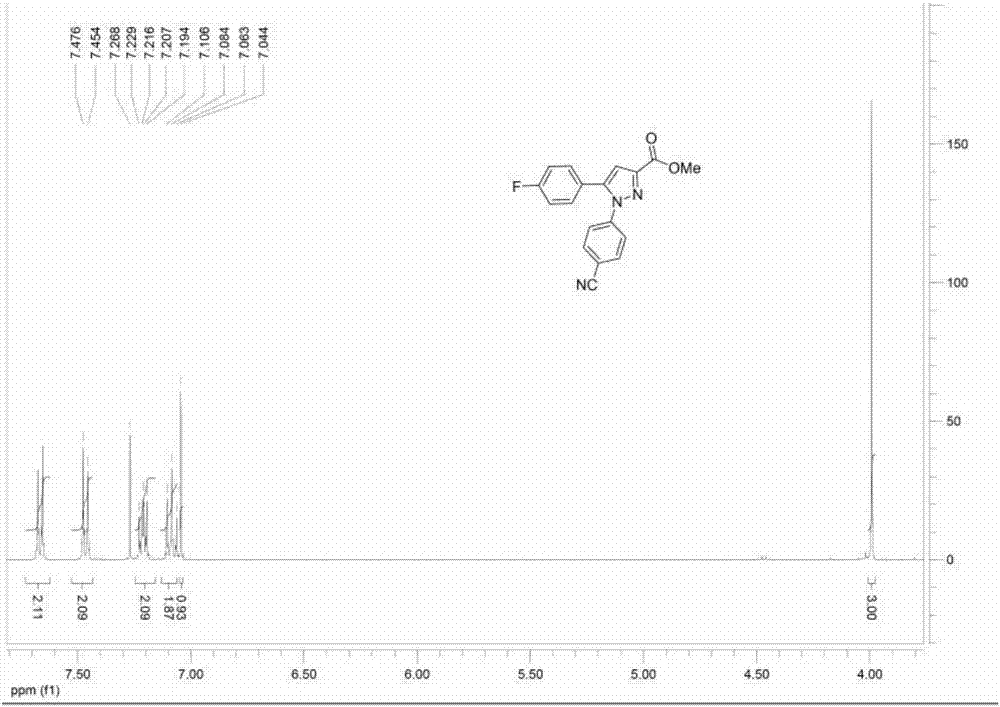
[0062] The synthetic process of compound (2) is the same as that of compound (1), except that the raw material reactants used are compound 2a and 4'-cyanophenylhydrazine, with a yield of 85% and a purity of 95%. . The detection results of compound (2) are ...
Embodiment 3
[0064] Embodiment 3: the synthesis of compound (3)
[0065] (1) Synthesis process of compound 3a
[0066]
[0067] The synthesis process of compound 3a is the same as that of compound 1a, except that the raw materials used are 100 ml of anhydrous methanol, 0.02 mol of sodium metal, 0.02 mol of diethyl oxalate, and 0.01 mol of 2-acetylphenanthrene. The purity of the obtained compound 3a was 90%, and the yield was 85%. The detection results of compound 3a are as follows: 1 HNMRδ: 9.3(s, 1H); 8.80(d, 1H, J=8.0Hz, Ar-H); 8.13-7.65(m, 7H, Ar-H); 7.30(s, 1H, pyrazole-H); 3.99 (s,3H,-OCH3); ESI-MS: m / z 329.1[M+Na] + .Such as Figure 5 shown.
[0068] (2) Synthetic process of compound (3)
[0069]
[0070] The synthetic process of compound (3) is the same as that of compound (1), except that the raw material reactants used are compound 3a and 4'-cyanophenylhydrazine, and the yield of compound (3) is 80%. , with a purity of 95%. The detection results of compound (3) are a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com