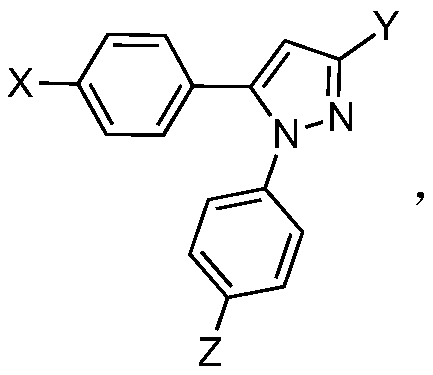
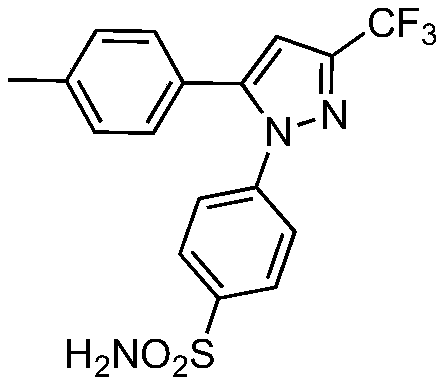
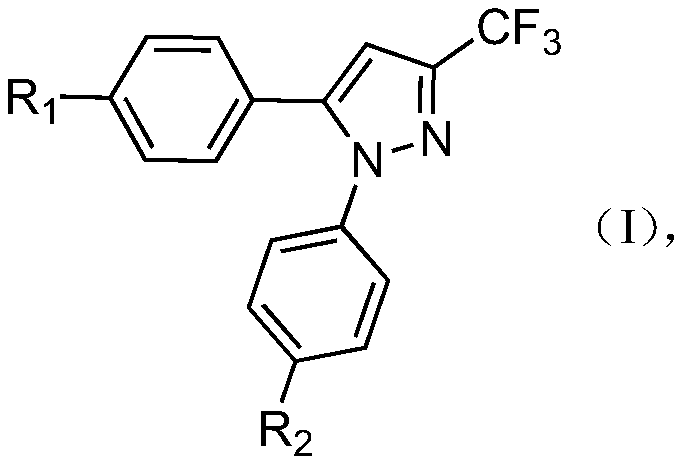
Application of 1,5-diaryl-3-trifluoromethylpyrazoles in the control of agricultural fungal diseases
A technology for trifluoromethylpyrazoles and fungal diseases, applied in the field of pesticides, can solve the problems of no antifungal drug reports, etc., and achieve the effect of broad-spectrum high-efficiency bactericidal activity and unique structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 compound (1)
[0029] a. Add 4.5 grams of sodium metal to 70 ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add 14 g of p-fluoroacetophenone and 17 g of ethyl trifluoroacetate (120 mmol), after the dropwise addition, heat up Reflux for 24 hours, concentrate to a yellow viscous liquid, add 2mol / L hydrochloric acid to adjust the pH to 2-3, extract with ethyl acetate, dry over magnesium sulfate, and evaporate the solvent to obtain a yellow oil 1-p-fluoro-4,4 , 4-trifluorobutanol-1,3 dione 21g, yield 90%.
[0030] b. Add 23.4g 1-p-fluoro-4,4,4-trifluorobutane-1,3dione and 19.g (mmol) p-carboxyphenylhydrazine hydrochloride to a round bottom flask filled with absolute ethanol , stirred and refluxed for 24 hours, solids were precipitated, filtered, and recrystallized from ethanol to obtain 28 g of the target compound, with a yield of 80%.
Embodiment 2
[0031] The preparation of embodiment 2 compound (2)
[0032] a. Add 4.5 grams of sodium metal to 70 ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add 13.5 g (100 mmol) of p-methylacetophenone and 17 g (120 mmol) of ethyl trifluoroacetate, drop After the addition, heat up and reflux for 24 hours, concentrate to a yellow viscous liquid, add 2mol / L hydrochloric acid to adjust the pH value to 2-3, extract with ethyl acetate, dry over magnesium sulfate, and evaporate the solvent to obtain a yellow oily substance 1-p Tolyl-4,4,4-trifluorobutan-1,3dione 20g, yield 86%.
[0033] b. Add 23g (100mmol) 1-p-tolyl-4,4,4-trifluorobutane-1,3-dione and 19g (100mmol) p-carboxyphenylhydrazine hydrochloride to a round-bottomed flask filled with absolute ethanol Salt, stirred and refluxed for 24 hours, a solid precipitated out, filtered, and recrystallized from ethanol to obtain 25.9 g of the target compound, with a yield of 75%.
Embodiment 3
[0034] The preparation of embodiment 3 compound (3)
[0035] a. Add 4.5 grams of sodium metal to 70 ml of anhydrous methanol, cool to room temperature after the reaction is complete, slowly add 15 g of p-methoxyacetophenone and 17 g (120 mmol) of ethyl trifluoroacetate, after the addition is complete , heated and refluxed for 24 hours, concentrated to a yellow viscous liquid, added 2mol / L hydrochloric acid to adjust the pH value to 2-3, extracted with ethyl acetate, dried over magnesium sulfate, and obtained yellow oily 1-p-methoxybenzene after distilling off the solvent 22.6 g of 4,4,4-trifluorobutan-1,3-dione, yield 92%.
[0036] b. Add 18.514g 1-p-methoxyphenyl-4,4,4-trifluorobutane-1,3-dione and 24.6g (100mmol) p-carboxyphenylhydrazine salt to a round bottom flask filled with absolute ethanol Salt 19g (100mmol), stirred and refluxed for 24 hours, a solid precipitated out, filtered, and recrystallized from ethanol to obtain 31.4g of the target compound, with a yield of 87%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com