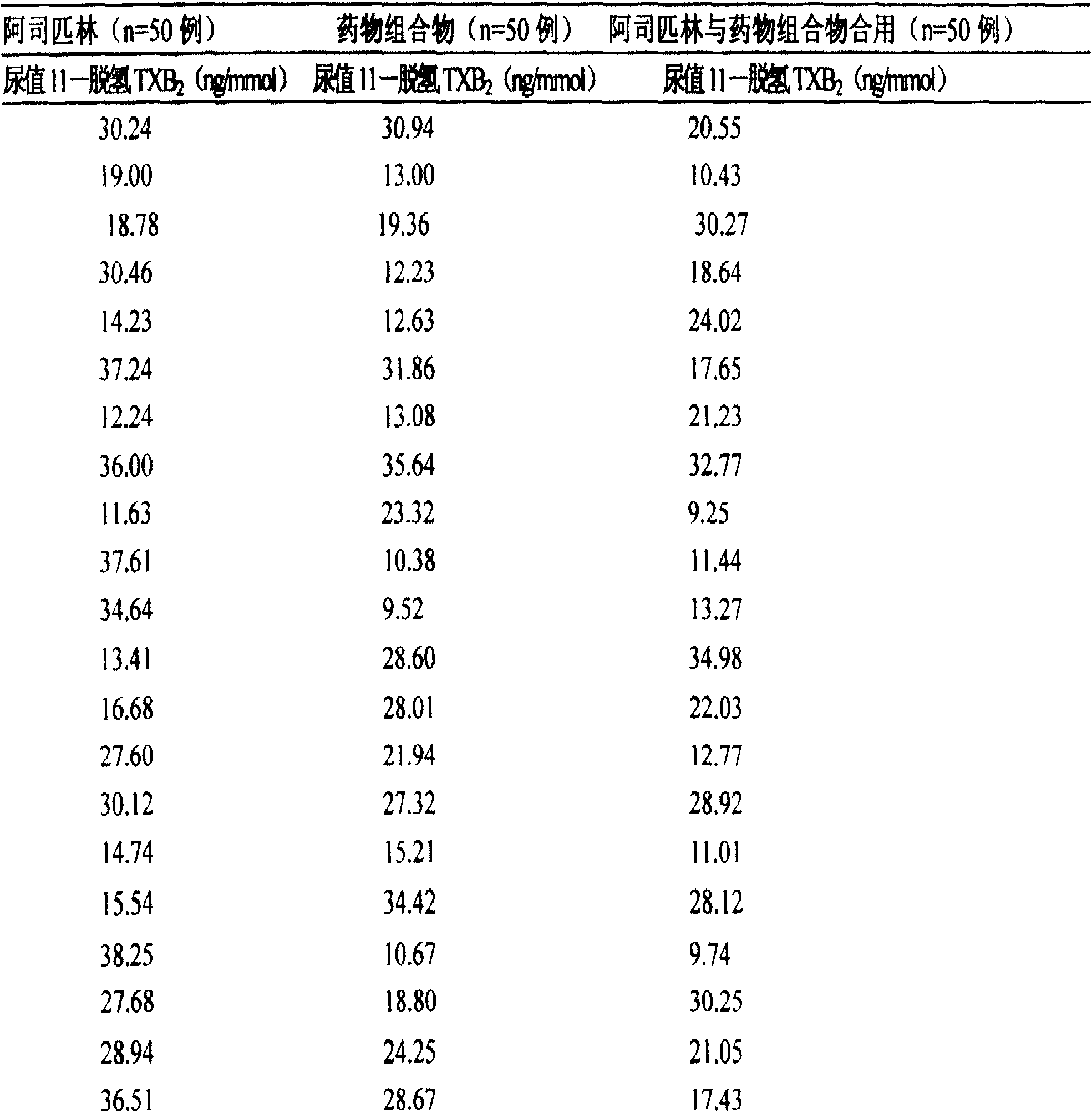
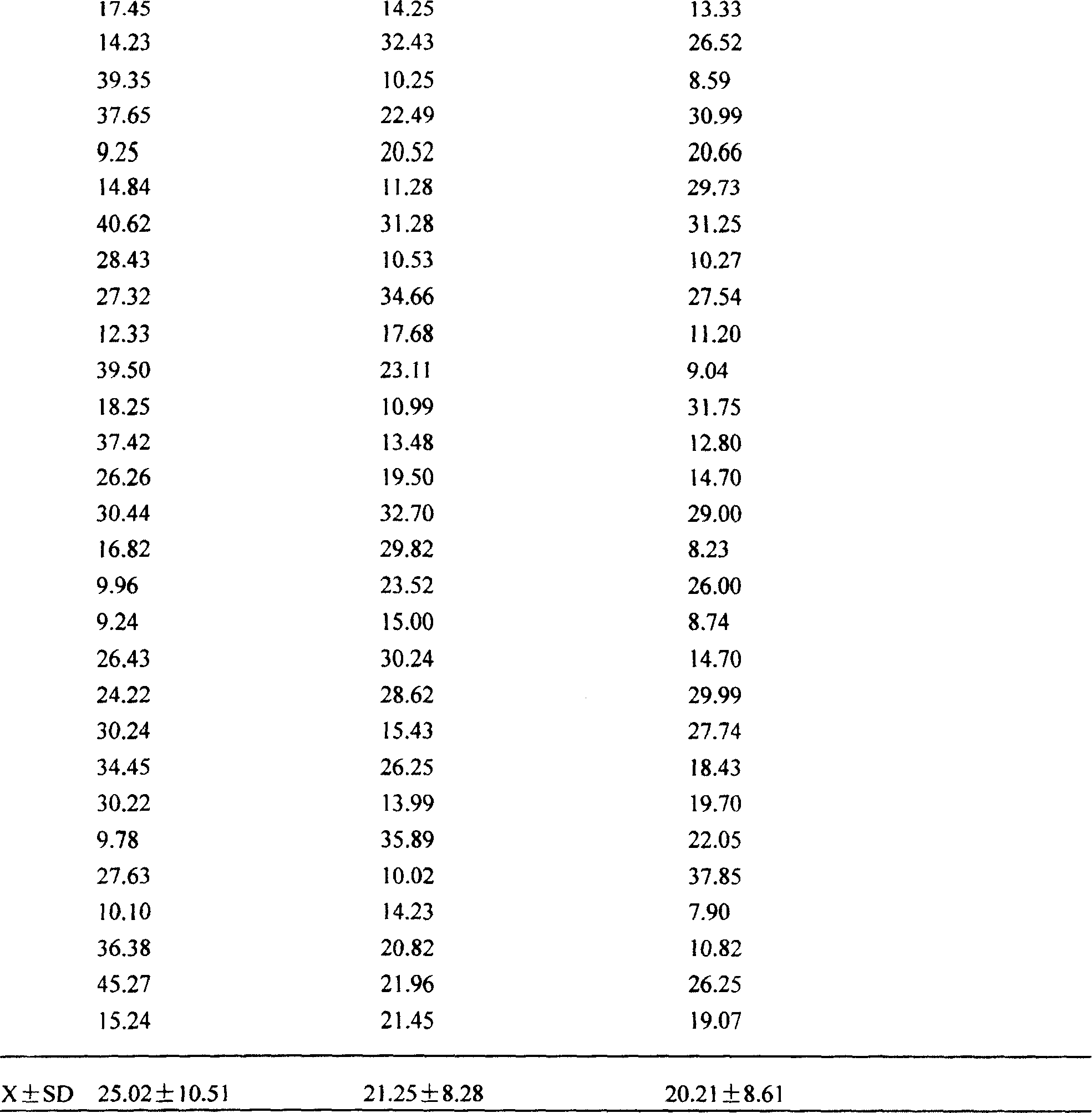
Application of pharmaceutical composition in the process for preparing medicine to treat aspirin resisting disease
A composition, aspirin technology, applied in the field of medicine, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] a. Take 450g of salvia miltiorrhiza, 141g of notoginseng, and 8g of borneol;
[0054] b. Extract Salvia miltiorrhiza 3 times in the following manner: heat and reflux with ethanol for 1.5 hours, filter, recover ethanol, concentrate the filtrate to form a concentrated solution with a relative density of 1.30 (55-60°C); heat and reflux the remaining medicinal materials with 50% ethanol for 1.5 hours, filter;
[0055] Continue to add water to reflux and heat the medicinal material for 2 hours, and filter. Combine the second and third extracts, recover ethanol, concentrate to a relative density of 1.40 (55-60°C), combine with the concentrated solution extracted for the first time, and make an infusion with a relative density of 1.35-1.39 (55-60°C). paste.
[0056] c. Grinding Panax notoginseng, mixing the above-mentioned concentrated salvia miltiorrhiza extract, drying into granules.
[0057] d. Grind borneol into fine powder, mix with the above granules, and make 1000 pi...
Embodiment 2
[0059] a. Take 450g of salvia miltiorrhiza, 141g of notoginseng, and 8g of borneol;
[0060] b. Extract Salvia miltiorrhiza 3 times in the following manner: heat and reflux with ethanol for 1.5 hours, filter, recover ethanol, concentrate the filtrate to form a concentrated solution with a relative density of 1.30 (55-60°C); heat and reflux the remaining medicinal materials with 50% ethanol for 1.5 hours, filter;
[0061] Continue to add water to reflux and heat the medicinal material for 2 hours, and filter. Combine the second and third extracts, recover ethanol, concentrate to a relative density of 1.40 (55-60°C), combine with the concentrated solution extracted for the first time, and make an infusion with a relative density of 1.35-1.39 (55-60°C). paste.
[0062] c. Grinding Panax notoginseng, mixing the above-mentioned concentrated salvia miltiorrhiza extract, drying into granules.
[0063] d. Grind borneol into fine powder, mix with the above granules, make 1000 pieces...
Embodiment 3
[0065] a. Take 480g of salvia miltiorrhiza, 130g of notoginseng, and 7g of borneol;
[0066] b. Extract Salvia miltiorrhiza 3 times in the following manner: heat and reflux with ethanol for 1.5 hours, filter, recover ethanol, concentrate the filtrate to form a concentrated solution with a relative density of 1.30 (55-60°C); heat and reflux the remaining medicinal materials with 50% ethanol for 1.5 hours, filter;
[0067]Continue to add water to reflux and heat the medicinal material for 2 hours, and filter. Combine the second and third extracts, recover ethanol, concentrate to a relative density of 1.40 (55-60°C), combine with the concentrated solution extracted for the first time, and make an infusion with a relative density of 1.35-1.39 (55-60°C). paste.
[0068] c. Grinding Panax notoginseng, mixing the above-mentioned concentrated salvia miltiorrhiza extract, drying into granules.
[0069] d. Grind borneol into fine powder, mix with the above granules, make 1000 pieces,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com