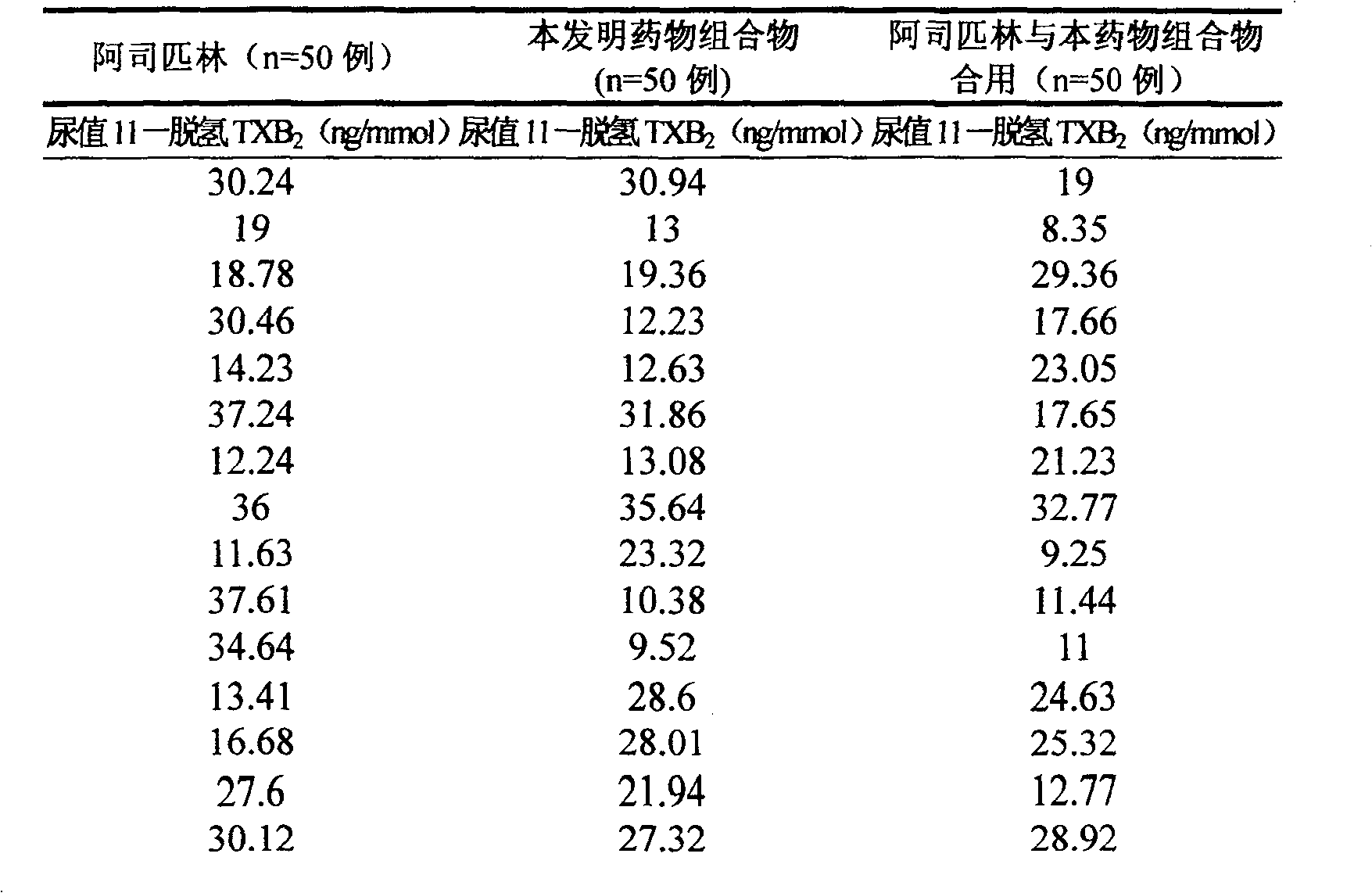
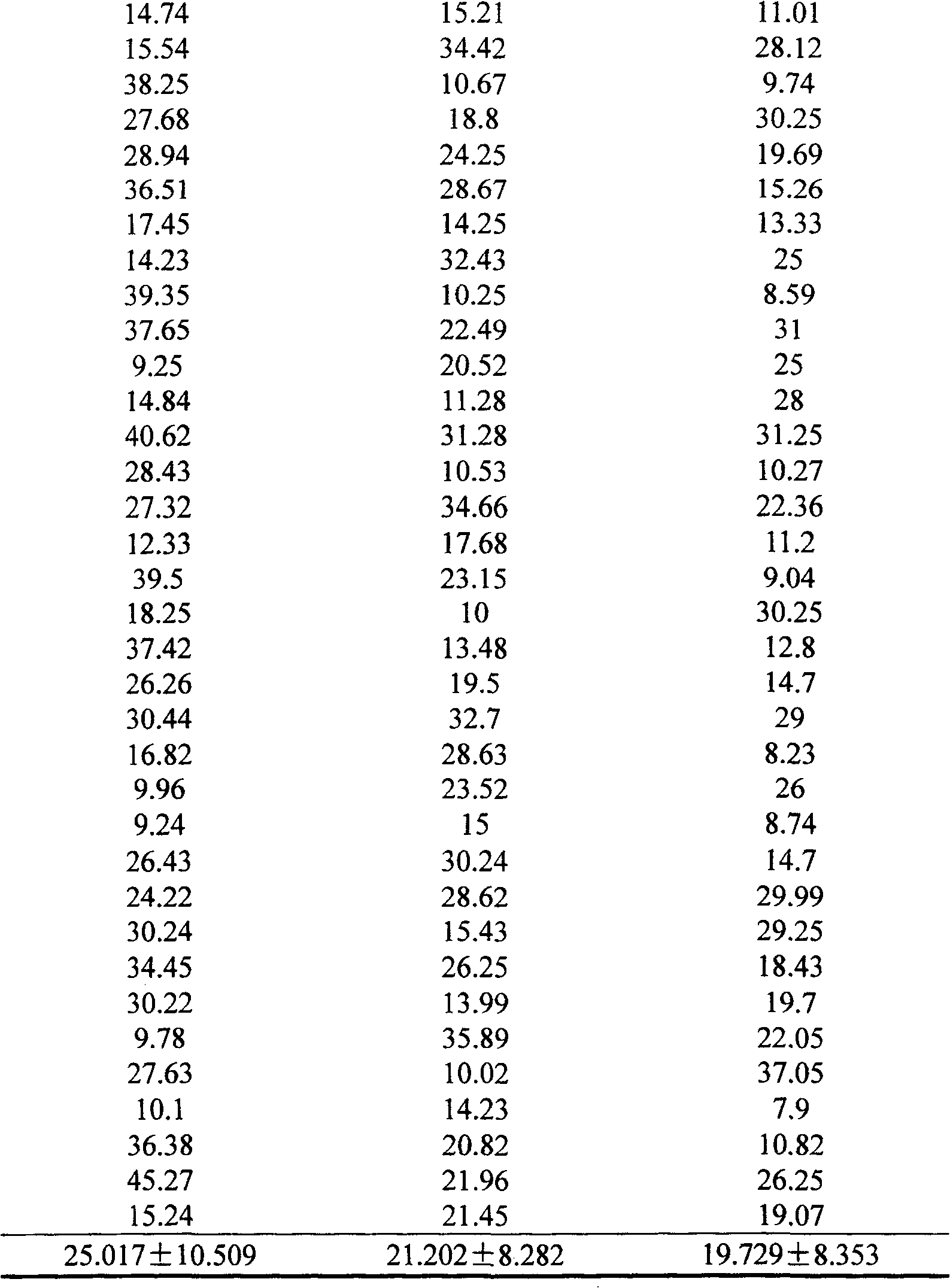Application of pharmaceutical composition in the process for preparing medicine to counteract aspirin resisting medicine
A technology of aspirin and composition, which is applied in the application field of preparing anti-aspirin resistance drugs, and can solve problems that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Take the Radix Paeoniae Alba in the amount of raw materials, add water and decoct 2-4 times, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.20-1.50 (50°C), dry, powder into fine powder, and then add the Ginseng stem and leaf saponins and 20-80 weight units of stevioside fine powder (sweetener) and 1-10 weight units of starch are granulated with 60%-80% ethanol, dried, and packed into capsules (each About 0.25g of medicine is filled in the capsule), and the product is obtained.
Embodiment 2
[0052] Take the Radix Paeoniae Alba in the amount of raw materials, add water and decoct 2-4 times, combine the decoction, filter, concentrate the filtrate under reduced pressure to a relative density of 1.20-1.50 (50°C), dry, powder into fine powder, and then add it to the amount of raw materials in turn Ginseng stem and leaf saponins and 20-60 weight units of stevioside fine powder (sweetener) and 1-10 weight units of starch are granulated with 60%-80% ethanol, dried, and magnesium stearate is added 1-5 weight units, mixed evenly, pressed into tablets (core weight 0.39 / tablet), and then coating the core with sugar (15-20kg of white granulated sugar for each kg of tablet core, 14-20kg of talcum powder, polished) , the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com