Transdermal therapeutic system with activatable oversaturation and controlled permeation promotion
一种治疗系统、渗透增强剂的技术,应用在经皮治疗系统领域,能够解决系统不可控制、稳定性问题、活性物质生物利用度降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
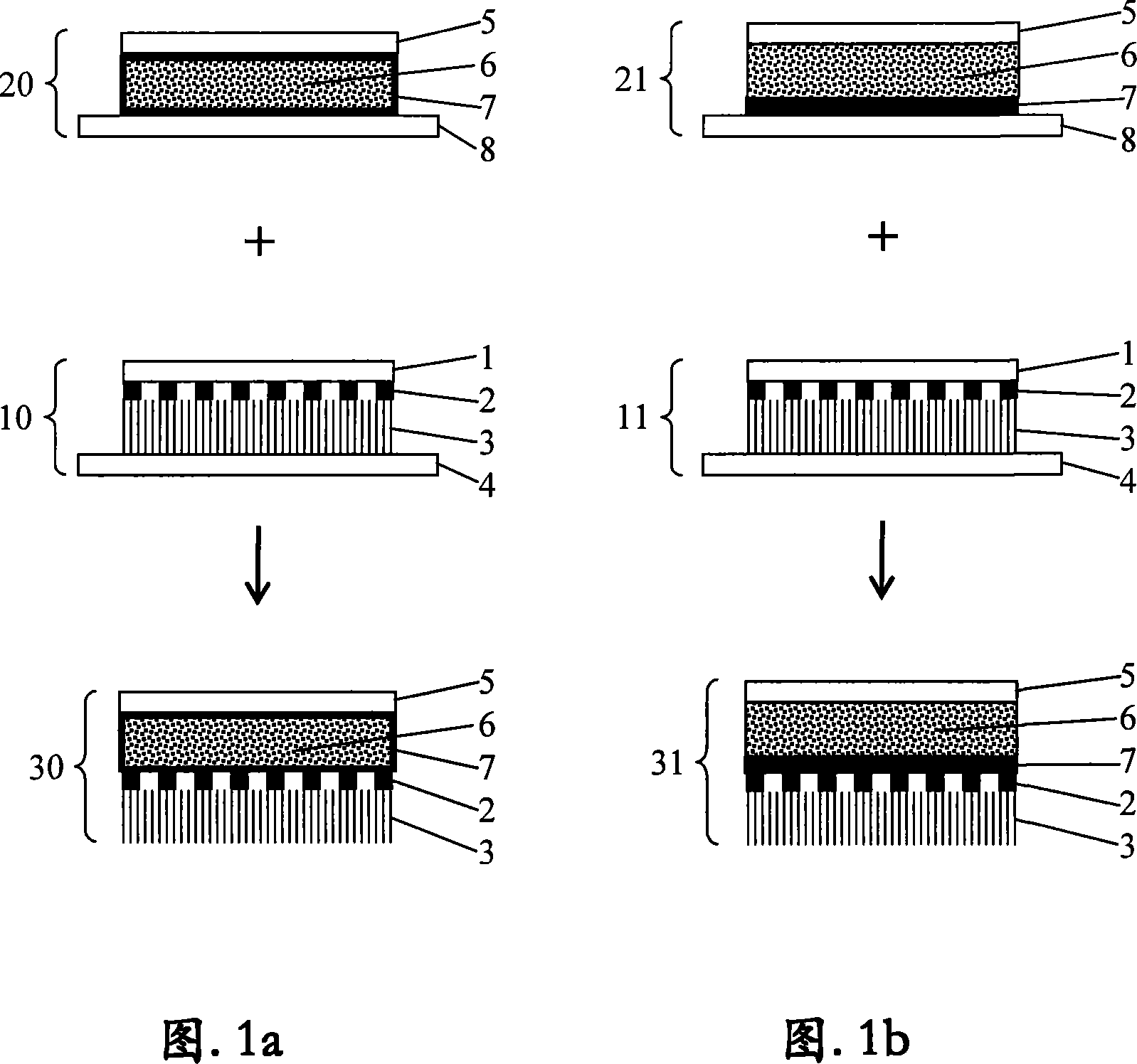
[0059] Example 1 : Manufacturing of active parts
[0060] With 54g containing the vinyl acetate of 40wt% and melt index be the EVA copolymer of 55 (EVATANE 40 / 55 ) into 80 g of a solvent mixture comprising 2 parts of 80 / 110 type specific boiling point gasoline and 1 part of propyl acetate and stirred at 50° C. by heating. After stirring for about 30 minutes, a viscous, colorless to slightly opaque solution was obtained. Next, 66g of binder resin Foral 85E was added and stirred again at 50°C until it completely dissolved (about 15 minutes). This yielded a 45.7%, low-viscosity, yellowish and slightly opaque solution (binder solution A), which remained as a stirrable binder solution even after cooling.
[0061] To prepare a self-adhesive active substance-containing matrix, 8.75 g of binder solution A are provided, to which 1.0 g of the lipophilic, sparingly water-soluble medical active substance moxonidine base are added in portions while stirring. The preparation was th...
Embodiment 2
[0066] Example 2 : Preparation of reinforcement part including self-adhesive control film
[0067] To prepare the self-adhesive enhancer part (liquid reservoir system), first, the active substance-free adhesive solution A (Example 1) was spread onto siliconized polyethylene terephthalate with a wet layer thickness of 300 μm using a spatula. on the ester film. Then, the solvent was removed by drying in a drying cabinet at 50° C. for 30 minutes using an exhaust pipe. The solvent-free and active-substance-free adhesive film was then bonded with a 35 μm thick polyurethane film (Opraflex , from the Lohmann company in Germany) (according to the embodiment of the invention EB1) or with a 25 μm thick polypropylene film (Celgard X-20 , US Celanese Seperation Products) coverage. These films ultimately form the control film. Thereafter, the polyester film (Scotchpak No.1220 , from 3M Company in Germany) was placed on this Opraflex or Celgard film and made into a bag with a c...
Embodiment 3
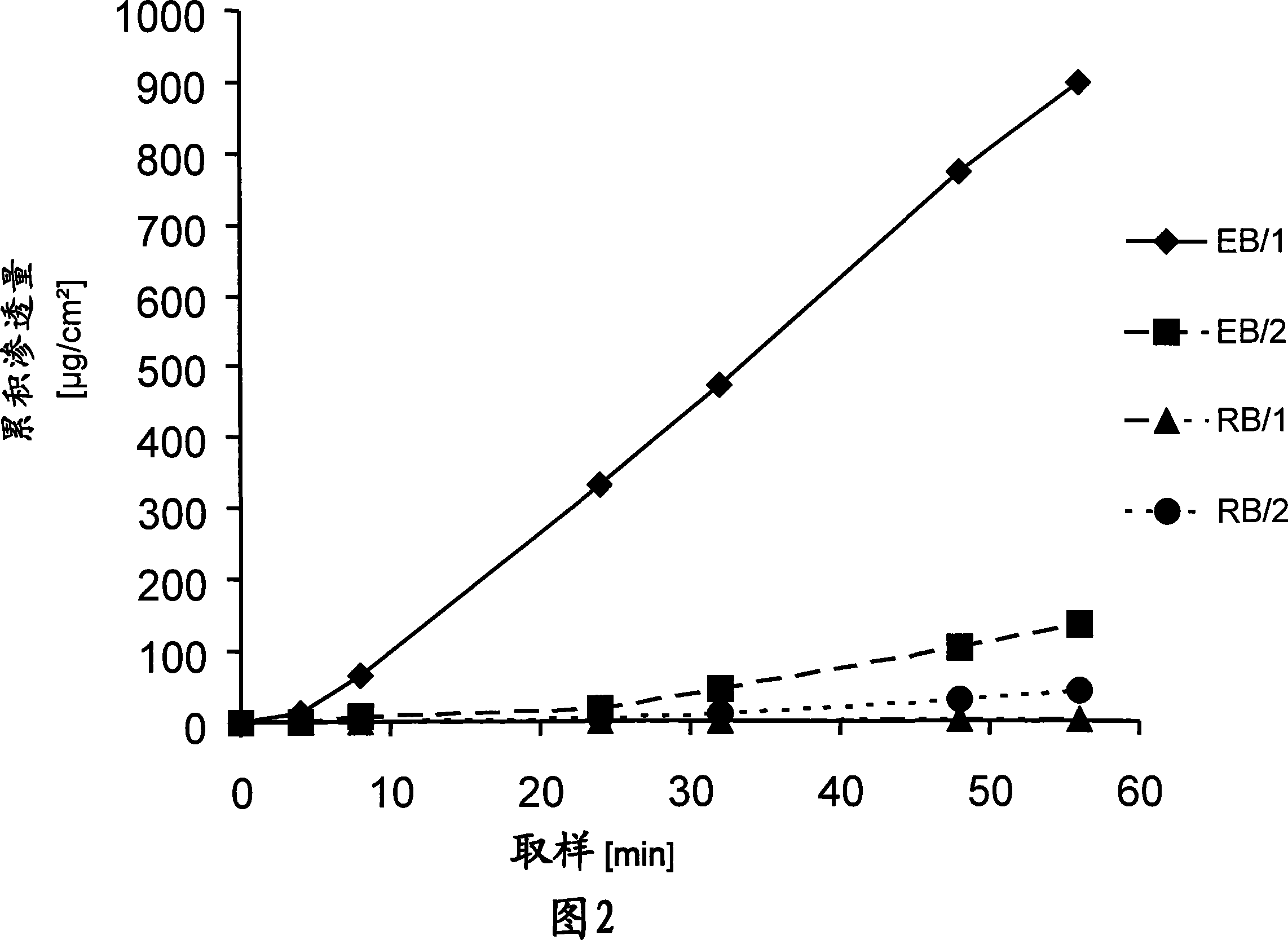
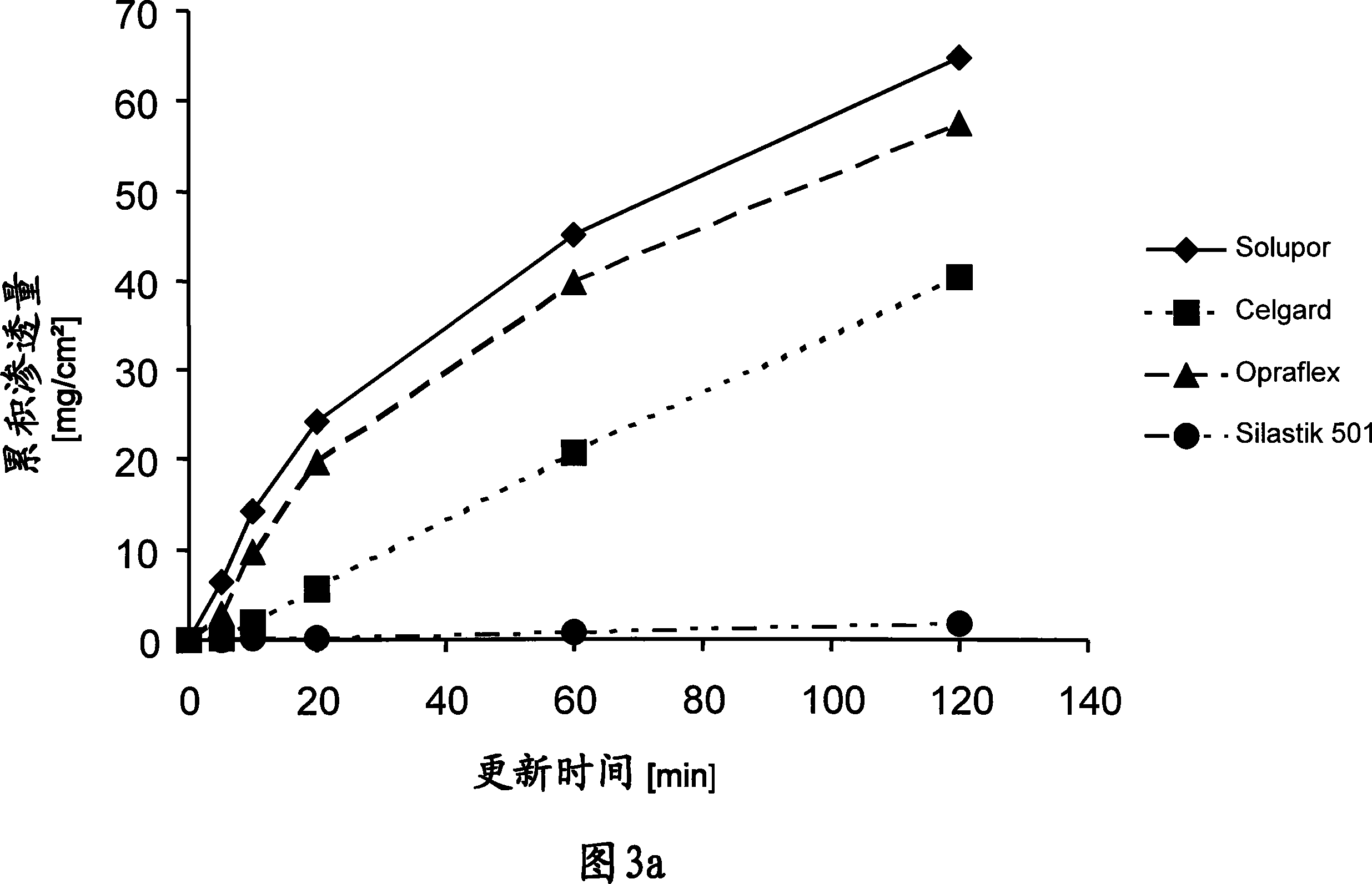
[0069] Example 3: Permeation diagram of TTS
[0070] In order to be able to test the permeation properties of the TTS according to the invention and to compare these properties with each other, permeation tests were carried out on an in vitro diffusion model of human full-thickness skin by using a modified Franz diffusion cell, the results of which are graphically represented in FIG. 2 .
[0071] The receiving medium used was the addition of 0.1% NaN at a temperature controlled to 32°C 3 Physiological sodium chloride solution as preservative.
[0072] The enhancer mixture used in the reservoir of the enhancer section was 300 mg of a mixture of ethanol:oleic acid:N-methylpyrrolidone in a ratio of 2:1.5:1.5 (v / v / v).
[0073] For the embodiment EB / 1 of the TTS according to the invention, after removal of the siliconized polyethylene terephthalate film, the reinforcing part EB1 according to Example 2 is applied as a cover patch to the TTS according to Example 1. on the active pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com