Process for production of carbapenem derivative and crystalline intermediate therefor
A crystallization and compound technology, applied in the field of preparation of carbapenem derivatives and crystallization of intermediates, can solve problems such as unoptimized implementation, unseparated crystallization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] (1) Preparation method of compound (I)
[0082] [化10]
[0083]
[0084] (In the formula, R is a hydrogen atom or a hydroxyl protecting group; R 1 Is a carboxy protecting group; R 2 Is an amino protecting group, Ph is phenyl).
[0085] After reacting compound (III) and compound (IV) or a pharmaceutically acceptable salt thereof in the presence of a base, the protective group of the hydroxyl group is deprotected as necessary to obtain compound (I), its pharmaceutically acceptable salt, and its solvate Or their crystals.
[0086] As the base, a secondary amine is used, and a secondary amine with a large steric hindrance is more preferable. Specifically, in NHR a R b Said, where R a And R b Each independently is an alkyl group or a phenyl group, etc., preferably R a And R b the same. The alkyl group is a linear or branched C1 to C10, preferably a C3 to C7 alkyl group, and more preferably a branched chain. As R a And R b , More preferably isopropyl, tert-butyl, isobutyl, pentyl a...
Embodiment 1
[0134] [化11]
[0135]
[0136] After dissolving the 2-propanol crystals of compound (Ia) described in WO2004 / 72073 with ethyl acetate (67ml) (3.340g, equivalent to 3.137g without solvent), the ethyl acetate and isopropyl were distilled off Alcohol gave ethyl acetate concentrate (4.551 g). Ethyl acetate (3.14 ml), benzyl alcohol (3.14 ml), and toluene (12.55 ml) were added thereto, and after stirring for 2 hours at room temperature, it was stirred for 1 hour at 5°C. The precipitated crystals were filtered, washed with toluene (6.26 ml), and air dried to obtain benzyl alcoholate crystals of compound (I-a) (3.668 g, equivalent to 3.037 g in solvent-free conversion, containing benzyl alcohol (1.0 mol)). Yield: 96.8%.
[0137] Mp.74.9℃
[0138] C 22 H 31 N 4 O 8 S 2 ·1.0C 7 H 8 O·0.2H 2 O element analysis
[0139] Theoretical value: C: 53.15, H: 6.06, N: 8.55, S: 9.79
[0140] Measured value: C: 53.19, H: 6.12, N: 8.65, S: 9.85
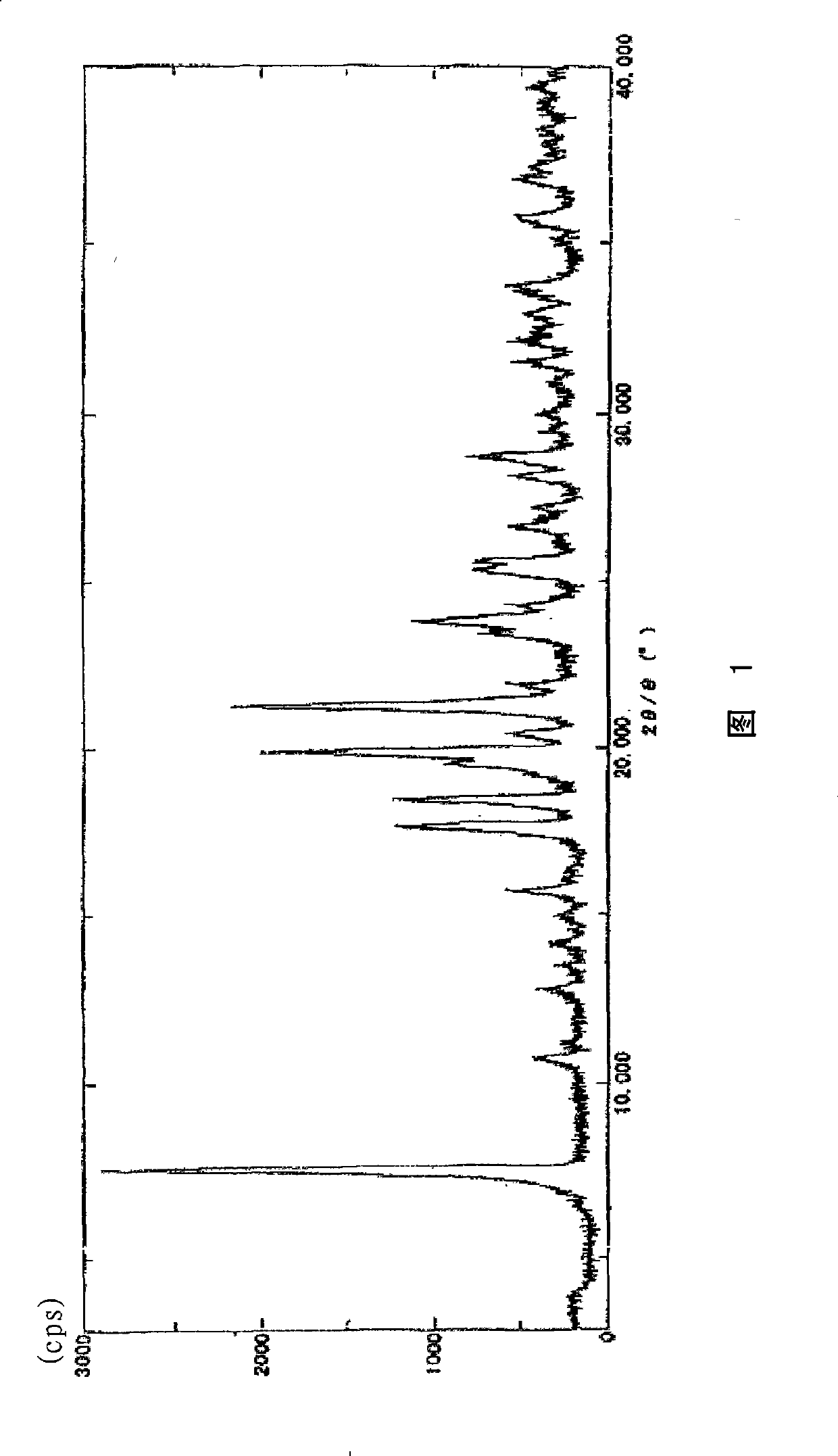
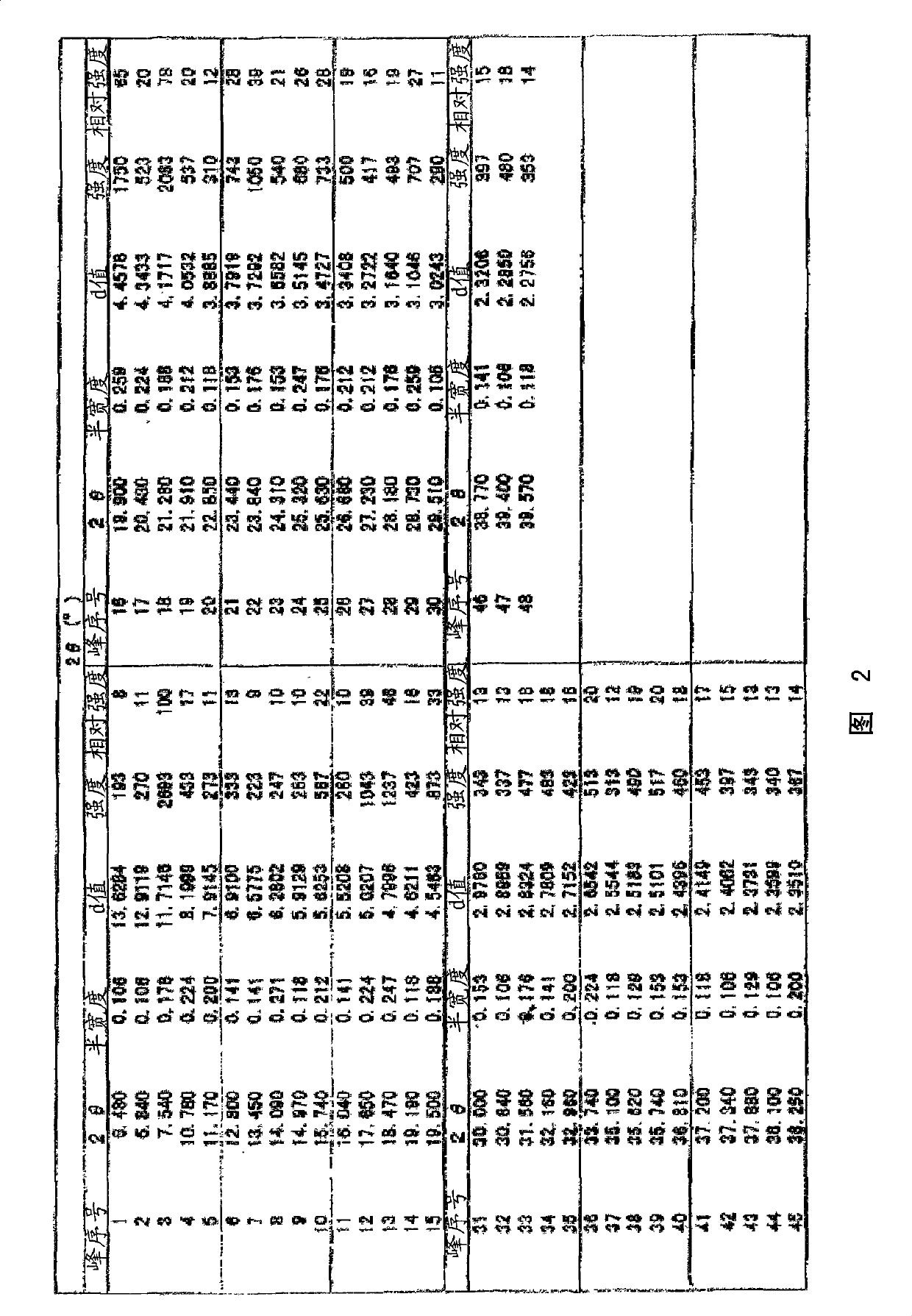
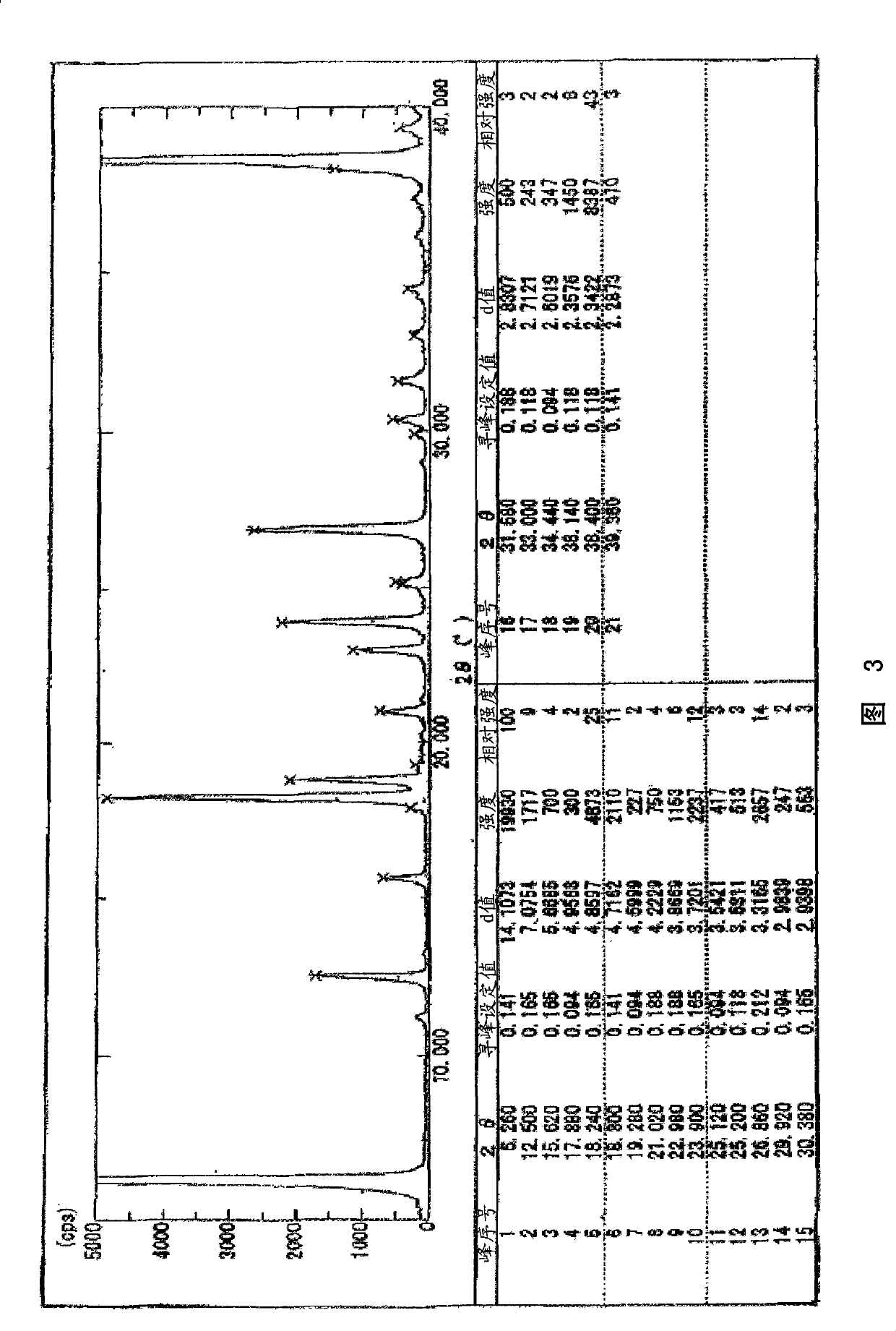
[0141] Powder X-ray diffraction: picture like figure 1 As s...
Embodiment 2
[0144] Amorphous and powdered compound (I-a) (100 mg) was dissolved in ethyl acetate (0.1 ml), benzyl alcohol (0.3 ml) was added, and the mixture was stirred at room temperature for 1 hour, and then left at 5°C for 2 days. The precipitated crystals were filtered and air-dried to obtain benzyl alcoholate crystals (79 mg) of compound (I-a) showing substantially the same powder X-ray pattern as in the case of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com