Aspartic acid lomefloxacin hydrate and preparation and uses thereof
A technology of lomefloxacin aspartate and hydrate, which is applied in the field of medicine, can solve the problems such as the preparation method and use of lomefloxacin aspartate hydrate which are not reported in the literature, and can be easily absorbed into the blood circulation. , good dissolution properties, good release properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
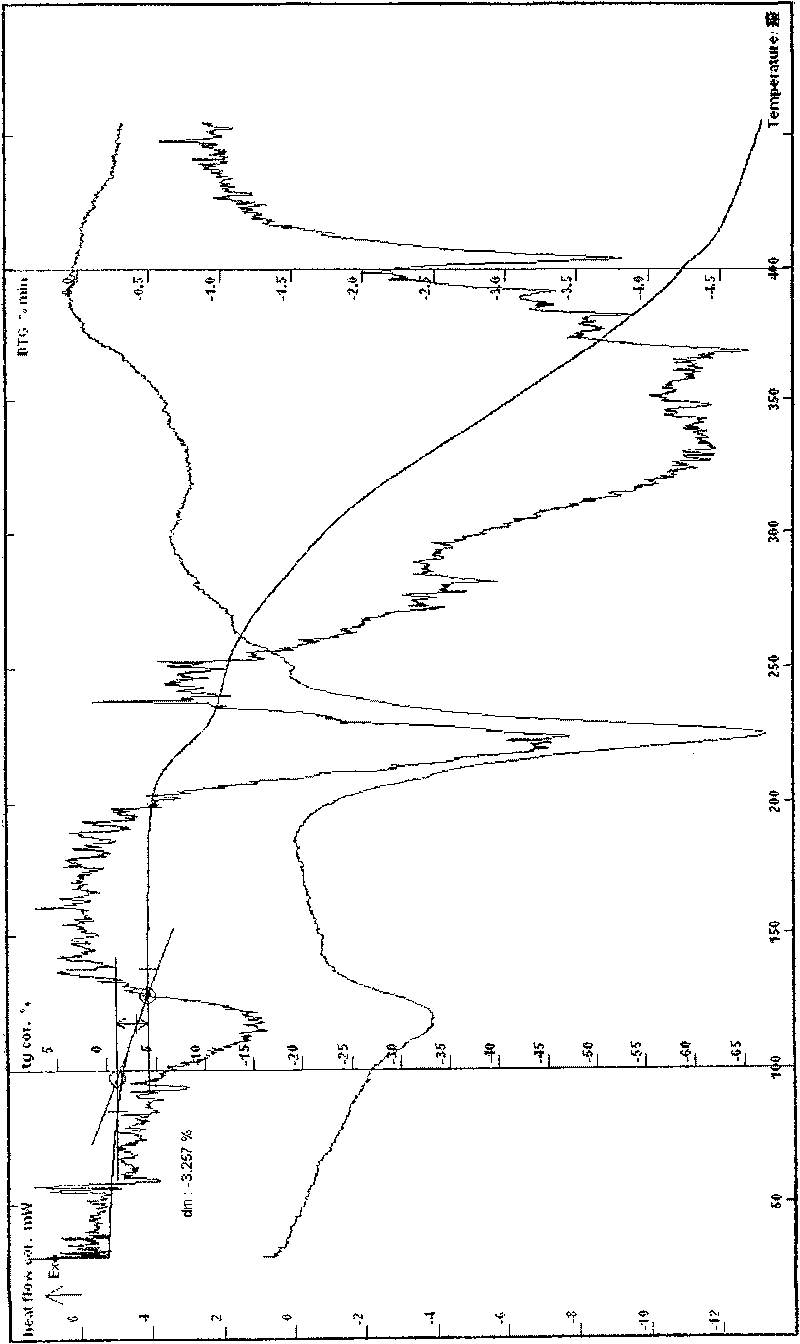
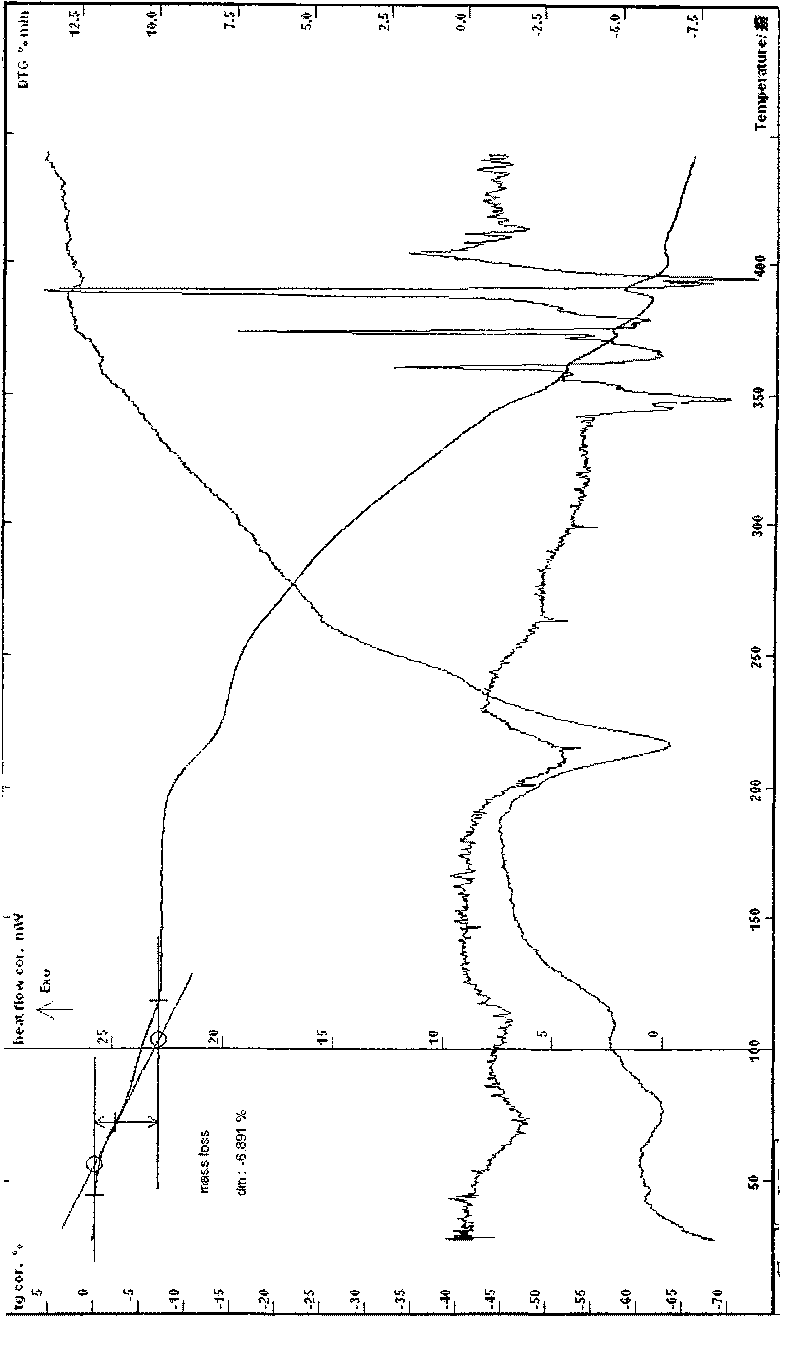
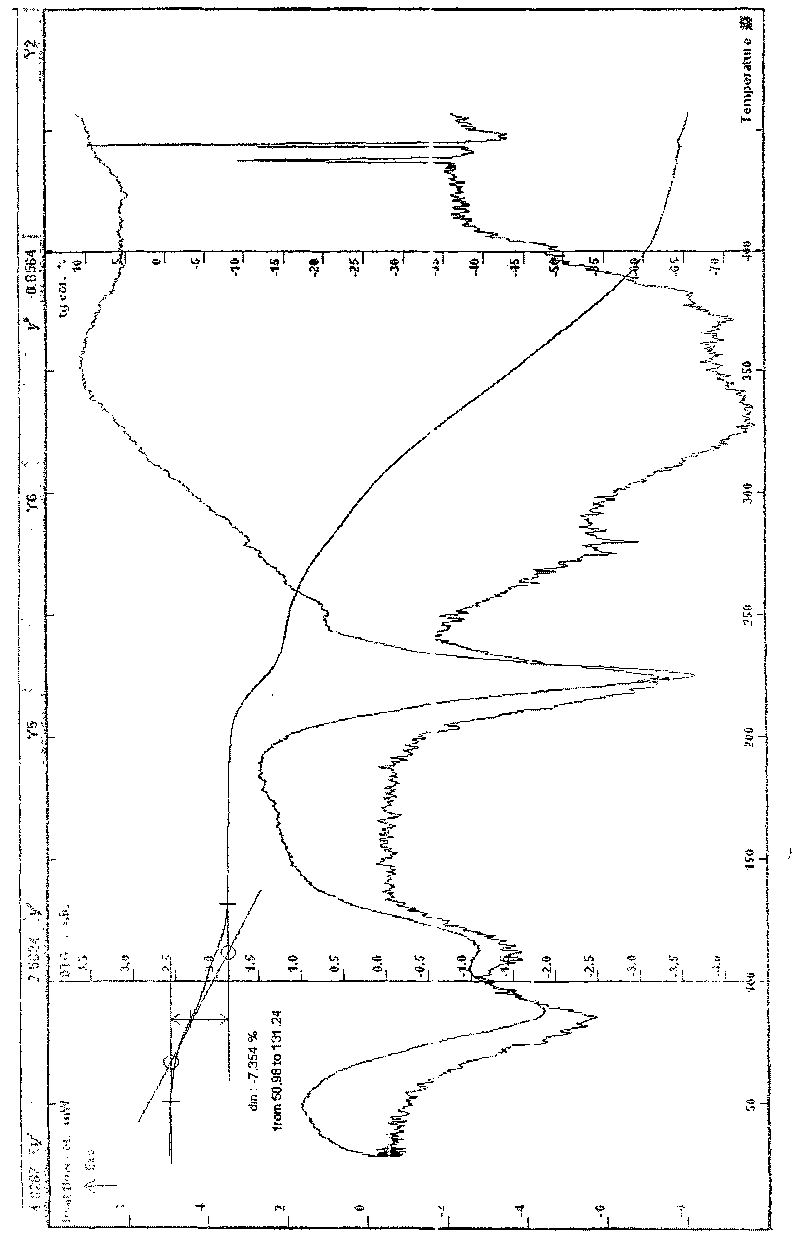
[0062]Example 1 In a three-necked flask, add 17.57g of lomefloxacin to 100ml of 95% ethanol, heat to reflux to dissolve, add 20ml of an aqueous solution of 6.66g of L-aspartic acid, stir at 40-100°C until reflux for 2h, and react After completion, slowly add 180ml of absolute ethanol, cool to below 0°C, wait for the solid to precipitate, filter, rinse the solid with ethanol several times, drain it, recrystallize with ethanol water, filter under reduced pressure, and dry at about 78°C for 6 hours to obtain Off-white crystalline powder 20.56g, easily soluble in water, MS (ESI, EI) m / e: 483 (M-18), 351 (M-133), melting point: 224.5-227.6°C, uncorrected; HPLC analysis: The retention time of the main peak of the sample is consistent with that of the main peak of the lomefloxacin reference substance; Karl Fischer's method measures moisture to be 3.69%, which is consistent with the result (theoretical value 3.58%) that the sample contains 1 water of crystallization; thermal analysis t...
Embodiment 2
[0065] Example 2 In a three-necked flask, add 10.21g of lomefloxacin and 3.87g of L-aspartic acid, add 10ml of water and 60ml of ethanol, heat to dissolve, stir continuously at 70-90°C to complete the reaction, and slowly add 120ml of absolute ethanol , cooled to about 0°C, waited for the solid to precipitate, filtered, the solid was rinsed with ethanol, drained, recrystallized from ethanol water, filtered under reduced pressure, dried at about 80°C for 4 hours to obtain 10.82g of off-white powder, MS (ESI, EI ) m / e: 483 (M-18), 351 (M-133), melting point: 223.3-226.9 ° C, uncorrected; HPLC analysis: purity 99.3%, the retention time of the main peak of the sample is consistent with that of the lomefloxacin reference substance. Moisture (Karl Fischer method): 3.76%, thermal analysis test (TG-DTG) shows: the weight loss of the sample is not obvious before 70 ° C, and the weight loss of the platform between 70 and 130 ° C is about 3.36%, which is consistent with the sample contain...
Embodiment 3
[0068] Example 3 Put 9.28g of lomefloxacin and 3.52g of L-aspartic acid into a reaction flask, add 60ml of water, heat to reflux, then control the temperature between 50-80°C, stir and react for 2h, after the reaction is completed, put it Freeze-dry, crystallize the obtained solid with about 22 times the volume of water, ethanol and isopropanol mixed solvent (2:10:10), cool, filter at low temperature, rinse with absolute ethanol, drain, and dry at about 50°C for 8 L-aspartic acid lomefloxacin dihydrate 9.76g, easily soluble in water, MS (ESI, EI) m / e: 483 (M-36), 351 (M-133), melting point: 215.5- 218.8°C, uncorrected; HPLC analysis: the retention time of the main peak of the sample is consistent with that of the lomefloxacin reference substance, and the water content measured by the Karl Fischer method is 6.79%, which is consistent with the result that the sample contains 2 crystal waters (theoretical value 6.92%) Consistent, thermal analysis test (TG-DTG): sample, 50 ~ 140 °...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com