Sugar-coated tablet
A preparation and sugar coating technology, applied in sugar-coated pills, pill delivery, medical preparations of inactive ingredients, etc., to achieve the effect of oxidation inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
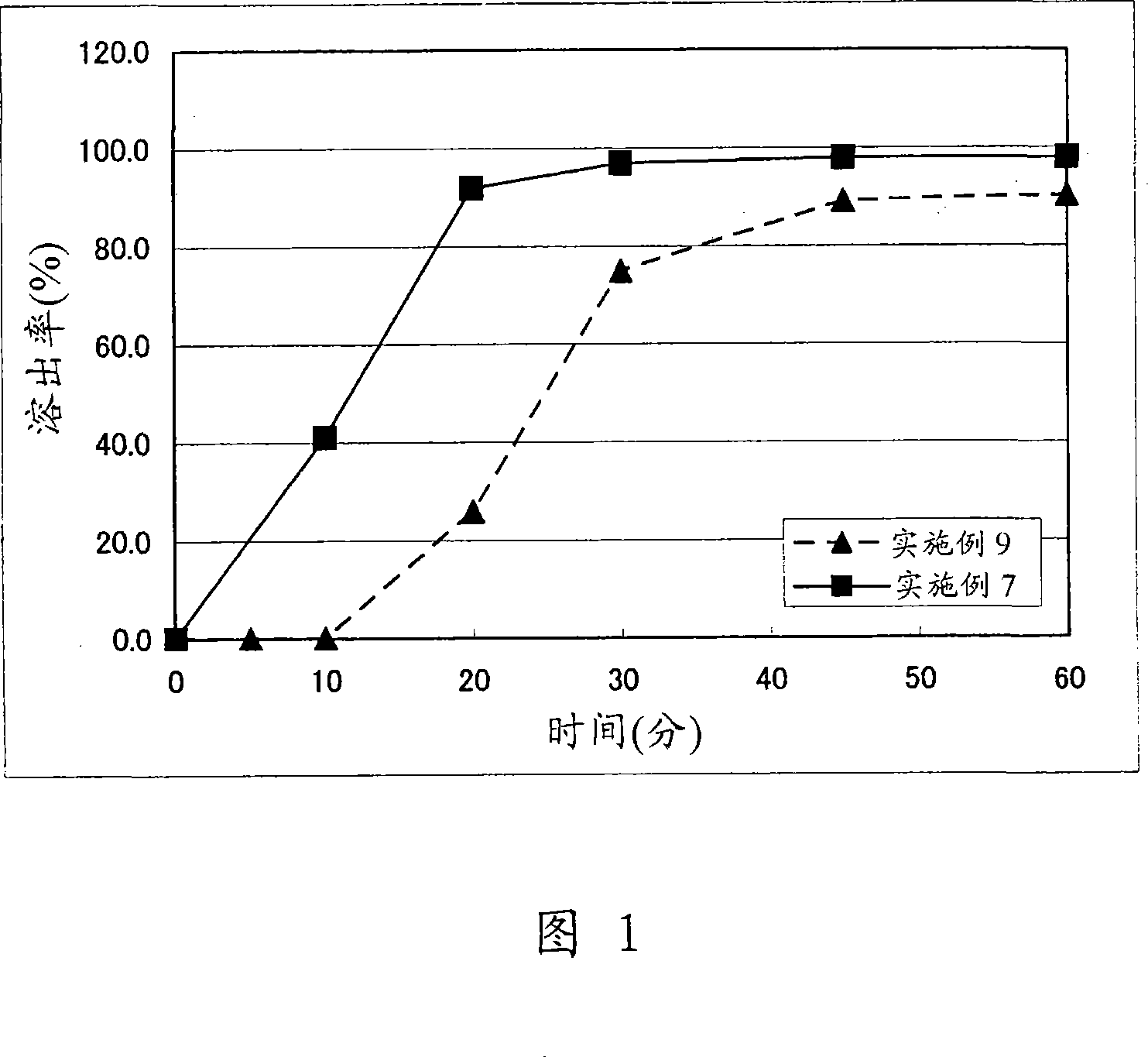
Image
Examples
Embodiment
[0075] Hereinafter, the present invention will be described in more detail by examples and the like, but they are not meant to limit the present invention.
[0076] In addition, the storage conditions in the following evaluation examples refer to storage in air, unless otherwise specified in the specification, such as "the oxygen concentration is 95% or higher". Further, in the storage conditions of the evaluation examples described below, no humidity adjustment was performed unless otherwise described about the humidity adjustment.
reference example 1
[0078] Compound A and sodium ascorbate were placed in the air at 40° C. and 75% RH for 1 month, respectively, and then their residual rates were measured. As a result, the residual rate of Compound A was 89.7% (W / W), and the residual rate of sodium ascorbate was 99.0% (W / W).
[0079]Quantification of Compound A was performed by the HPLC method under the following conditions.
[0080] Solvent: Acetonitrile
[0081] Measurement wavelength: 287nm
[0082] Column: CHIRALCEL OJ-R 4.6 mm×150 mm (manufactured by Daicel Chemical Industries, Ltd.)
[0083] Mobile phase: mixed solution of acetonitrile / 10mM ammonium acetate aqueous solution (16:9)
[0084] Column oven temperature: about 25°C
[0085] Sodium ascorbate was quantified by iodine titration (solvent: metaphosphoric acid solution (1→50), indicator: starch test solution).
Embodiment 1
[0095] Formulations were prepared according to the recipe shown in Table 3. Sugar-coated tablets were obtained by spraying the sugar-coating solution to the tablets (330 g) obtained in Comparative Example 2 by using a pan coater (Hicoater 20, Freund Industrial Co., Ltd.) so as to obtain a coating of 170 mg per tablet , the sugar coating solution comprises erythritol (221g), talc (68g), gum arabic powder (34g) and microcrystalline cellulose (17g). At this time, adjust the product temperature to 35°C to 55°C for coating.
[0096] [table 3]
[0097] 25mg tablet
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com