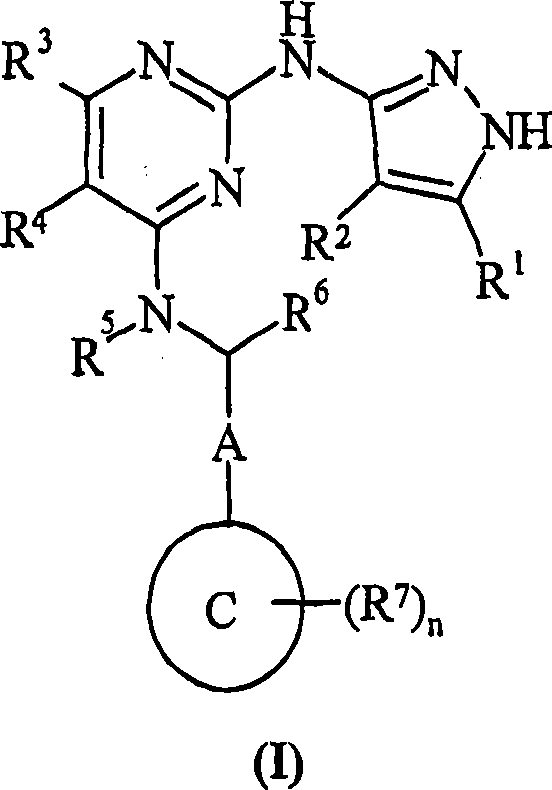
Pyrazolyl-amino-substituted pyrimidines and their use in the treatment of cancer
An amino and carbamoyl technology, applied in the field of new pyrazole derivatives, can solve problems such as doubtful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] (2R)-2-({5-chloro-2-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]pyrimidin-4-yl}amino)-2-(4-fluoro Phenyl)ethanol
[0242] To a microwave vial (Personal Chemistry) was added (2R)-2-[(2,5-dichloropyrimidin-4-yl)amino]-2-(4-fluorophenyl)ethanol (Method 1, 250 mg, 0.83 mmol), 5-cyclopropyl-1H-pyrazol-3-amine (204 mg, 1.66 mmol), DIPEA (0.17 mL, 0.97 mmol) and n-butanol (2 mL). The reaction mixture was heated to 160°C and maintained for 5 hours. Solvent was removed. The white solid was purified by semi-preparative HPLC (Gilson) to give the title compound (43 mg, 13%). 1 H NMR (300MHz, DMSO-d6) δ0.76(m, 2H), 0.97(m, 2H), 1.87(m, 1H), 3.73-3.85(m, 2H), 5.22(m, 2H), 7.13( m, 2H), 7.47 (m, 2H), 7.97 (d, 1H), 8.28 (s, 1H).
Embodiment 2-8
[0244] According to a method similar to Example 1, the following compounds were synthesized by reaction of the appropriate pyrimidine or quinazoline (the production method of which is also listed) with the appropriate amine.
[0245] implement
example
name
1 H NMR
SM1
SM2
2
(2R)-2-({2-[(5-cyclopropyl
-1H-pyrazol-3-yl) amino] pyrimidine
Pyridin-4-yl}amino)-2-(4-fluoro
Phenyl)ethanol
(DMSO-d 6 +D 2 O): 0.63 (m, 2H),
0.93(m, 2H), 1.82(m, 1H), 3.67
(m, 2H), 4.98-5.17(m, 1H),
5.61-5.70(m, 1H), 6.30-6.62(m,
1H), 7.09(m, 2H), 7.32(m, 2H),
7.68-7.86 (m, 1H)
Method 2
5-cyclopropyl
-1H-pyrazole
-3-amine
3
(2R)-2-({2-[(5-cyclopropyl
-1H-pyrazol-3-yl)amino]-5
-(trifluoromethyl)pyrimidin-4-yl]
Amino}-2-(4-fluorophenyl) B
Alcohol
0.67(m, 2H), 0.94(m, 2H), 1.88
(m, 1H), 3.75H), 2H), 5...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap