Polypyrazole nitrogen heterocyclic compound as well as preparation and application thereof
A nitrogen-heterocyclic compound and polypyrazole technology, which is applied in the direction of analyzing materials through chemical reactions, organic chemistry, and material analysis through observation of the impact on chemical indicators, can solve environmental pH sensitivity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
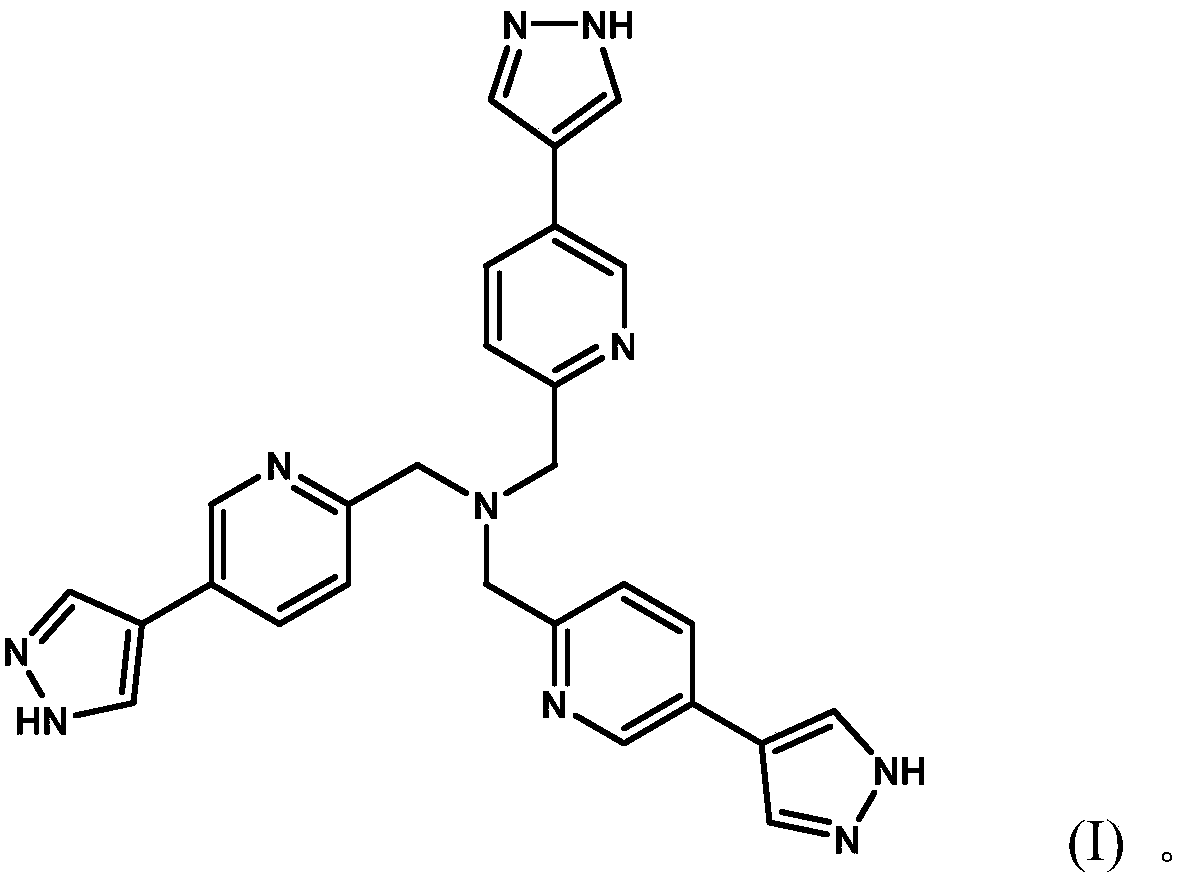
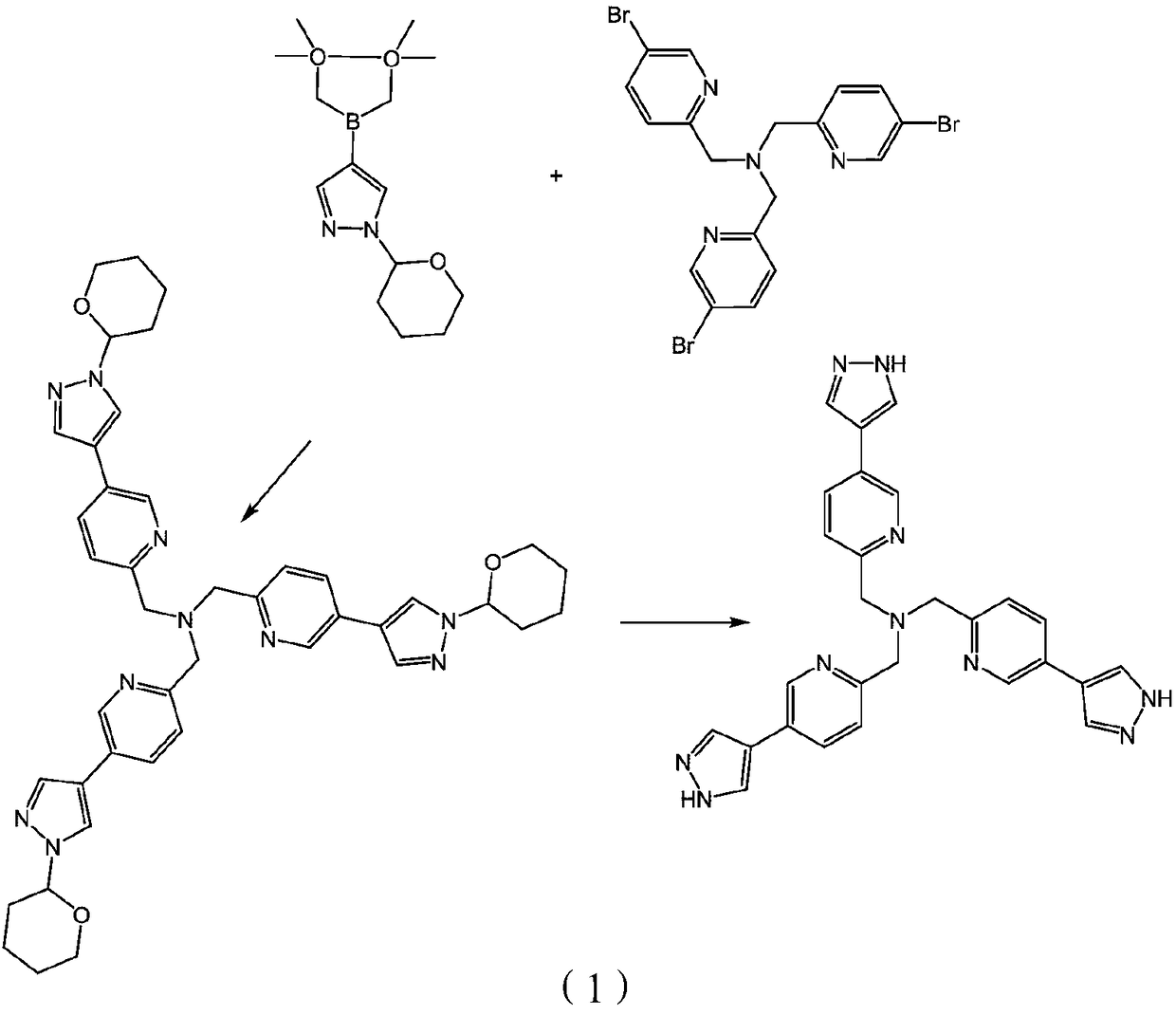
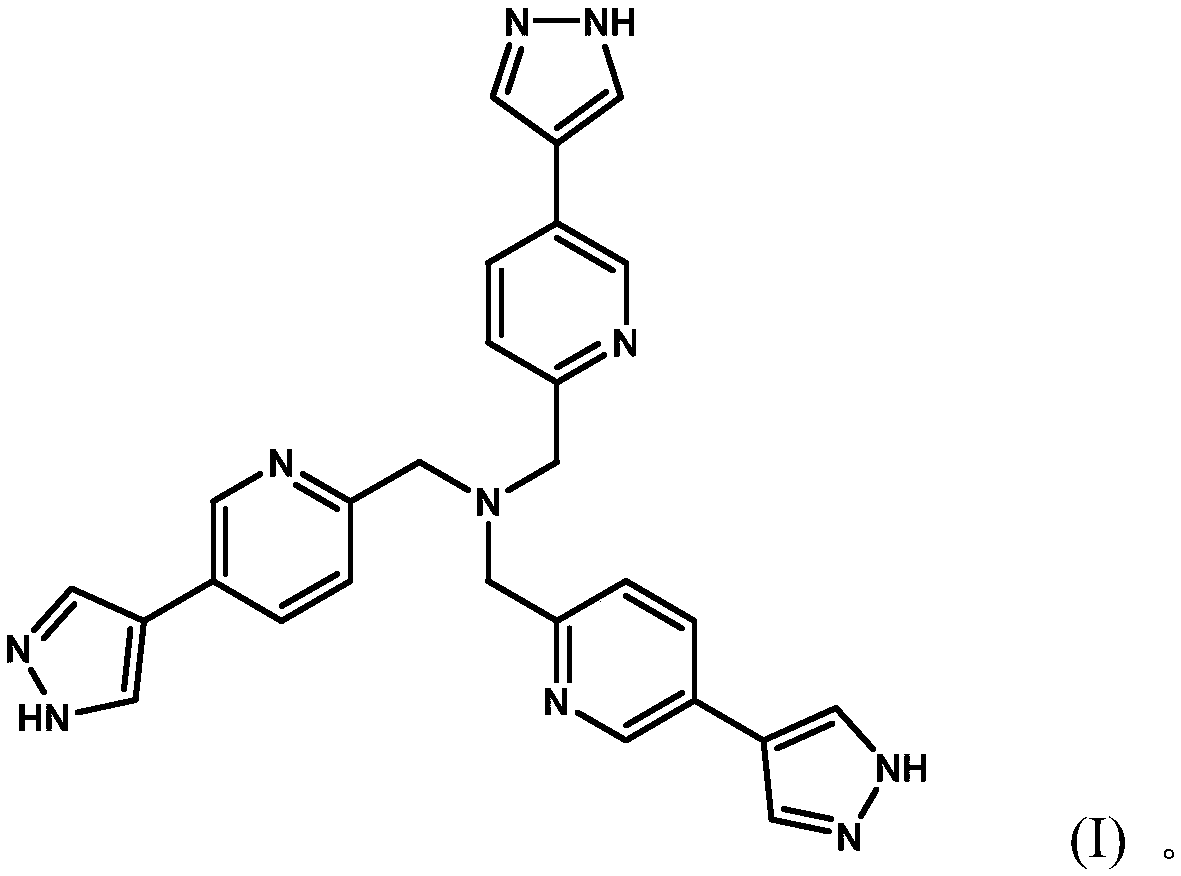
[0033] Embodiment 1: the synthesis of polypyrazole nitrogen-containing heterocyclic compound
[0034] Weigh tris[(5-bromopyridine)-2-methyl)]amine (552mg, 1.05mmol) and tetrakis(triphenylphosphine)palladium (244mg, 0.22mmol) in a 100mL three-necked flask, add 20mL n-butyl Alcohol and 10ml of water, install a reflux device, add 4mL of 2M potassium carbonate aqueous solution, add 1-(tetrahydropyran-4-yl)-1H-pyrazole-4-boronic acid pinacol under the protection of gas (nitrogen) The ester (1.18g, 4.19mmol) was stirred at 120°C for 72 hours.
[0035] The solvent was distilled off under reduced pressure, the resulting product was dissolved in dichloromethane, extracted three times with water, the organic layer was taken, and the solvent was removed; the product tri((5-(1-(tetrahydropyran-4-yl) -1H-pyrazol-4-yl)pyridin-2-yl)methyl)amine. The product tris((5-(1-(tetrahydropyran-4-yl)-1H-pyrazol-4-yl)pyridin-2-yl)methyl)amine was dissolved in methanol 20ml, and dilute hydrochloric ac...
Embodiment 2
[0040] Embodiment 2: the selectivity of the ion recognition body of the present invention to halide ions:
[0041] Take 4.8 mg of the compound of formula (I) prepared by the present invention as an ion recognition body, add the ion recognition body into a sample bottle, and prepare a 5 mM turbid aqueous solution. Prepare various halogen ion sodium salt solutions with deionized water, the concentration is 5mM, respectively NaCl, NaBr, NaI, NaF aqueous solution, and other non-halogen ion aqueous solutions, respectively Na 2 SO 4 , NaNO 3 , Na 2 CO 3 , Na 3 PO 4 , NaC 2 o 4 , the concentration is 5mM.
[0042] Add this series of ionic aqueous solutions to the system, only the addition of halide ions causes the obvious solution to turn yellow and clear. This result shows that the ion recognizer of the present invention has a high degree of recognition for halogen ions.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap