Preparation method of chlorantraniliprole and intermediate thereof
A technology for chlorantraniliprole and intermediates, which is applied in the chemical field of pesticide synthesis, can solve the problems of low molar yield of chlorantraniliprole intermediates and the like, achieves reduction of solvent usage, high product quality, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
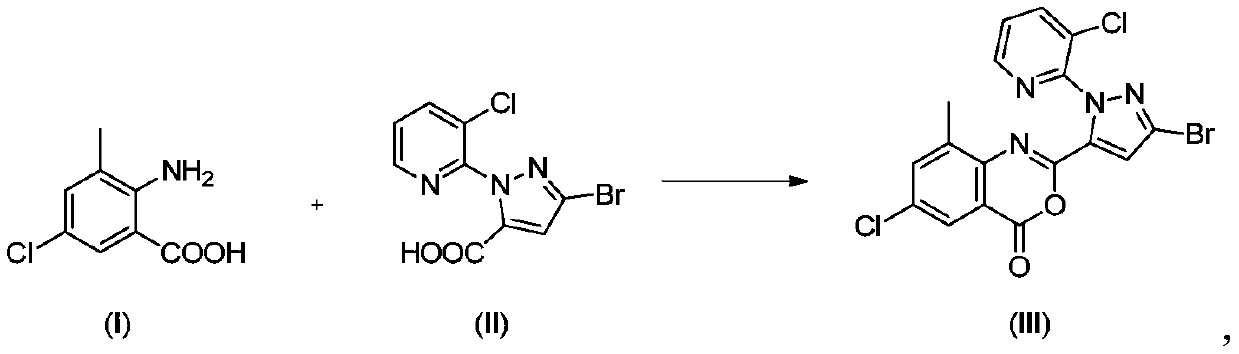
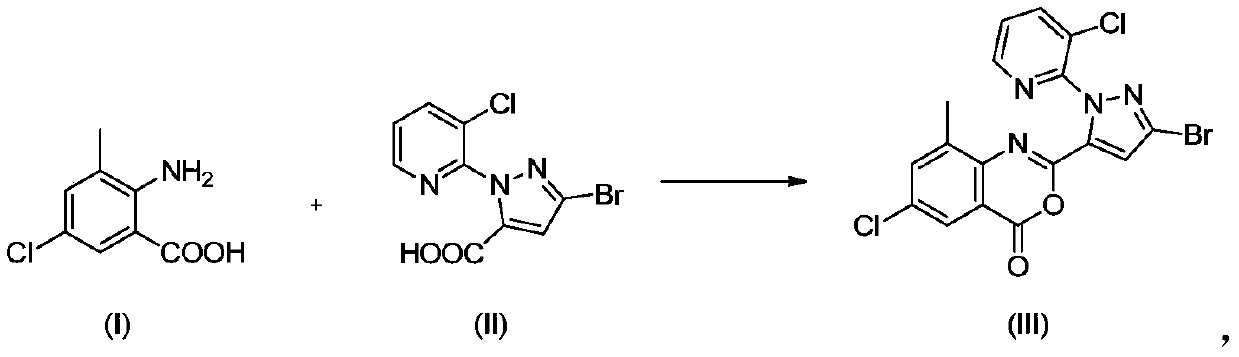
[0024] According to an exemplary embodiment of the present invention, the preparation method of the chlorantraniliprole intermediate mainly comprises: in the presence of a base and a solvent, formula I compound 2-amino-5-chloro-3-methylbenzoic acid (hereinafter Hereinafter abbreviated as B-2) and formula II compound 3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid (hereinafter referred to as K acid) are mixed together to form material one ; Methanesulfonyl chloride (hereinafter referred to as MsCl) is mixed with material one reaction again, obtains formula III compound 2-[3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazol-5-yl] -6-Chloro-8-methyl-4H-3,1-benzoxazin-4-one (abbreviated as BCPP hereinafter), which is the intermediate of chlorantraniliprole (III).
[0025] Among them, the preparation method of chlorantraniliprole intermediate BCPP also includes a post-treatment step of filtration, the resulting filter cake is crude BCPP, and the filtrate continues to be used as ...
Embodiment 1
[0036] Step A: Preparation of BCPP
[0037] Add K acid (200.0 g, 0.66 mol), B-2 (122.7 g, 0.66 mol) and 1 L of acetonitrile into a 2 L three-necked flask, and start mechanical stirring. Weigh 3-methylpyridine (320.2 g, 3.44 mol) into it, and stir at room temperature for about 5-10 min. The system was moved to a low temperature bath to start cooling down. When the internal temperature of the system is 0-2°C, start to slowly add MsCl (181.8g, 1.59mol) dropwise in total, keeping the internal temperature of the system not exceeding 5°C. After the dropwise addition, the temperature was naturally raised to room temperature, and the stirring reaction was continued until the reaction was complete. The filter cake and filtrate are obtained by filtration, the filter cake is the crude product of BCPP, and the filtrate is used as solvent recovery.
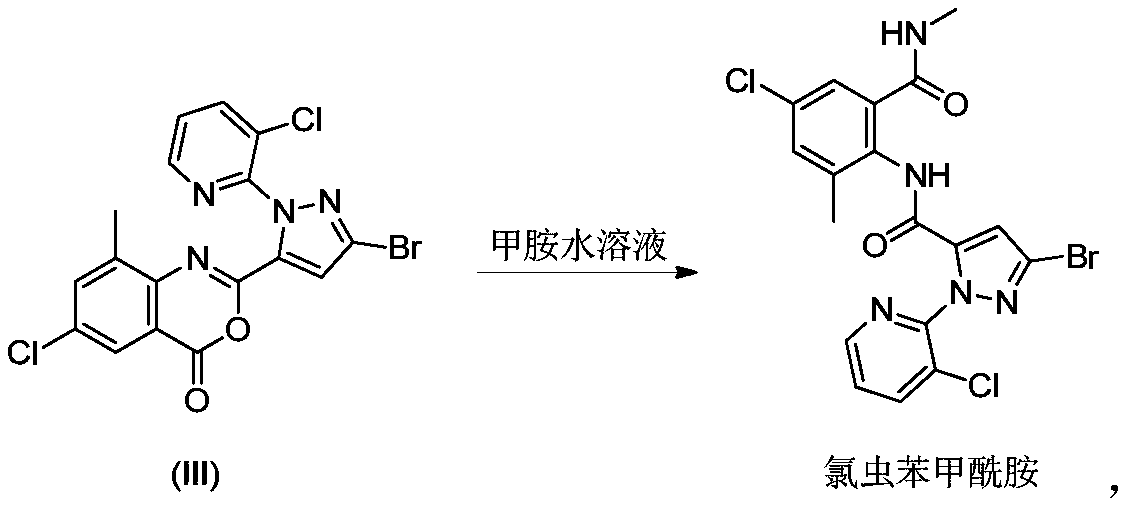
[0038] Step B: Preparation of Crude Chlorantraniliprole
[0039]Add 1 L of acetonitrile to the reaction flask containing the above-mentio...
Embodiment 2
[0043] Step A: Preparation of BCPP
[0044] Add K acid (200.0g, 0.66mol), B-2 (122.7g, 0.66mol) and solvent 1L respectively in 2L there-necked flask, this solvent is made up of filtrate 0.95L and acetonitrile 0.05L in step A among the embodiment 1, Turn on mechanical agitation. Weigh 3-methylpyridine (320.2 g, 3.44 mol) into it, and stir at room temperature for about 5-10 min. The system was moved to a low temperature bath to start cooling down. When the internal temperature of the system is 0-2°C, start to slowly add MsCl (181.8g, 1.59mol) dropwise in total, keeping the internal temperature of the system not exceeding 5°C. After the dropwise addition, the temperature was naturally raised to room temperature, and the stirring reaction was continued until the reaction was complete. The filter cake and filtrate are obtained by filtration, the filter cake is BCPP crude product, and the filtrate can still be used as a solvent for the recovery of the next batch.
[0045] Step B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com