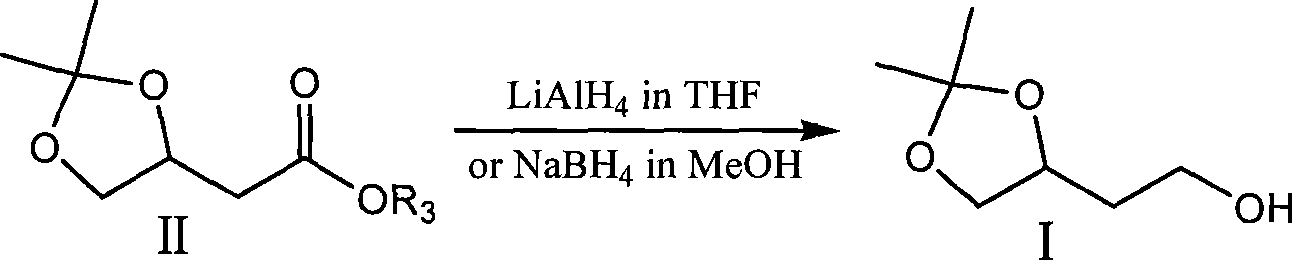Method for preparing 1,2-ketal protected-1,2,4-butanetriol
A technology of ketal protection and butanetriol, which is applied in the production of bulk chemicals and organic chemistry, can solve problems such as poor operability, increased cost, and unsuitability for large-scale production, and achieve high utilization rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
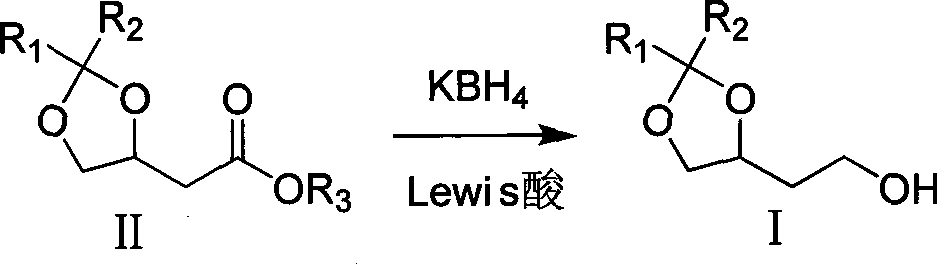
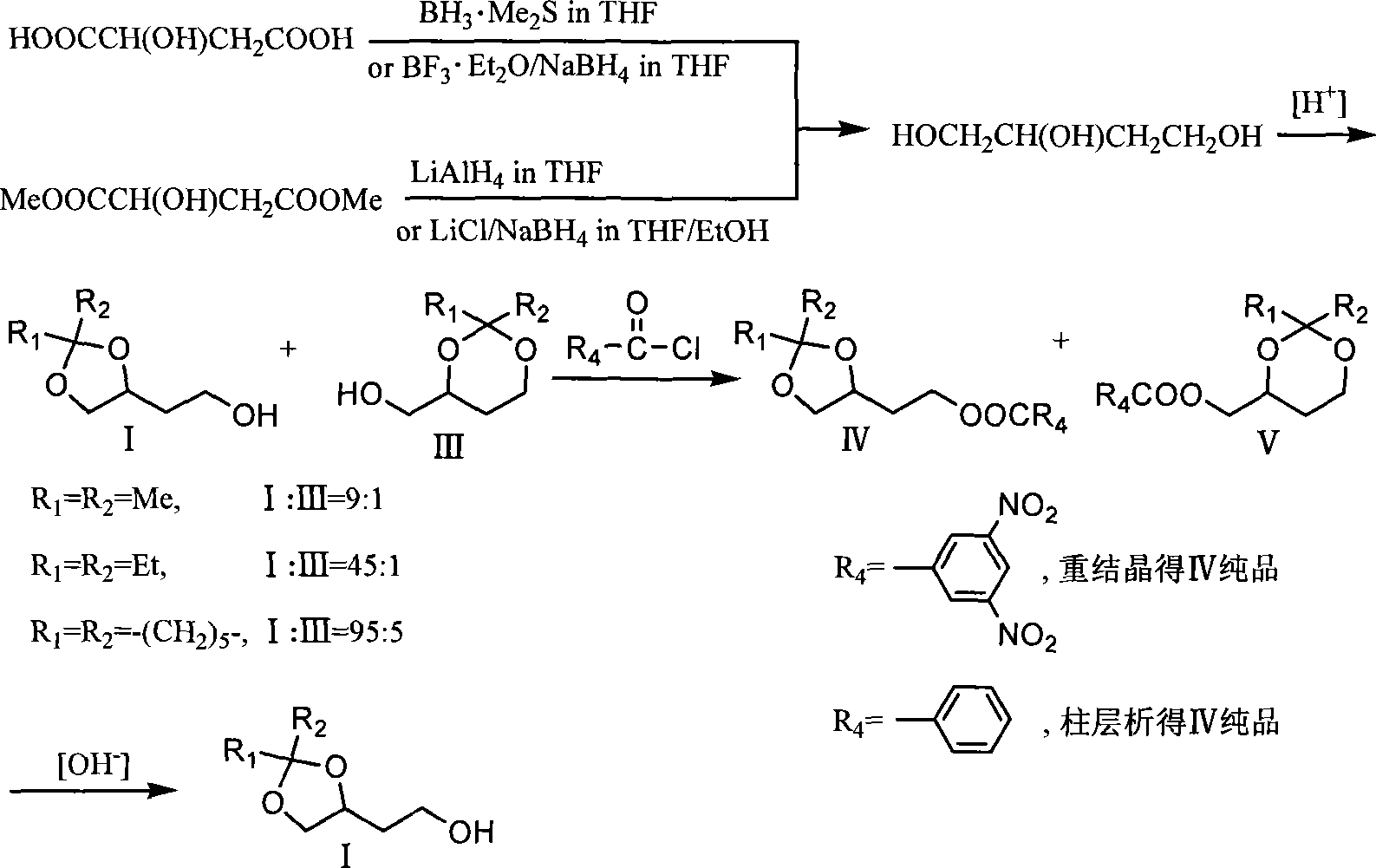
[0022] Add 100ml tetrahydrofuran and 3.27g KBH successively into a 250ml three-necked flask equipped with electromagnetic stirring, a thermometer and a reflux condenser 4 , 5.46g magnesium chloride, heated to reflux in an oil bath under the protection of nitrogen for 2 hours, then added 10.0g II dropwise, and the drop was completed in about 2 minutes. reaction, filter the reaction solution, wash the filter cake with a mixed solution of 40ml tetrahydrofuran and methanol (10:1), concentrate the filtrate to dryness, add 200ml methanol to dissolve the resulting residue and concentrate to dryness again, and dissolve the resulting residue with 200ml ethyl acetate , washed with saturated brine, anhydrous MgSO 4 Drying, solvent removal under reduced pressure obtains 7.58g colorless transparent oil product (S)-I[R 1 = R 2 =Me; GC: 97.8%; bp: 75-76°C / 2mmHg; 1 H-NMR (CDCl 3 , 400MH Z ): 1.35(3H,s), 1.42(3H,s), 1.82(2H,m), 2.39(1H,s), 3.58(1H,dd), 3.78(2H,t), 4.08(1H,dd) , 4.25(1H, ...
Embodiment 2
[0024] Add 80ml tetrahydrofuran, 2.61gKBH successively into a 250ml three-necked flask equipped with electromagnetic stirring, a thermometer and a reflux condenser 4 , 4.37g magnesium chloride, 10.0g (R)-II (R 1 = R 2 = Me,R 3 =Et), drop it in about 2 minutes, continue to reflux for 2 hours, then change to an ice-water bath to cool to an internal temperature of 5-10°C, and carefully add 10ml of methanol to quench the reaction. Filter the reaction solution, wash the filter cake with a mixed solution of 60ml tetrahydrofuran and methanol (10:1), concentrate the filtrate to dryness, add 50ml methanol to dissolve the residue and concentrate again to dryness, and dissolve the residue with 200ml ethyl acetate , washed with saturated brine, anhydrous MgSO 4 Drying, solvent removal under reduced pressure obtains 7.31g colorless transparent oil product (S)-I(R 1 = R 2 =Me).
Embodiment 3
[0026] Add 150ml tetrahydrofuran, 5.88g KBH successively into a 500ml four-necked bottle equipped with electromagnetic stirring, a thermometer and a reflux condenser 4 , 4.40g lithium chloride, 15.0g (S)-II (R 1 = R 2 = R 3 =Me), heated to reflux in an oil bath under the protection of nitrogen for 3 hours, then changed to an ice-water bath to cool to an internal temperature of 5-10°C, carefully added 50ml of saturated brine to quench the reaction, extracted with 3×200ml of ethyl acetate, combined the organic layers and Anhydrous MgSO 4 Drying, solvent removal under reduced pressure obtains 11.33g colorless and transparent oil product (S)-I(R 1 = R 2 = Me).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com