Low molecular weight heparin composition and uses thereof
一种低分子量肝素、重均分子量的技术,应用在含有效成分的医用配制品、有机活性成分、过敏性疾病等方向,能够解决不是常规试验或易于实施、昂贵等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0460] The present invention is further illustrated by the following examples, which should not be construed as limiting the present invention. The contents of all references, patents and published patent applications cited in this application are hereby incorporated by reference.
Embodiment
[0462] Method of producing M118-REH
[0463] production process
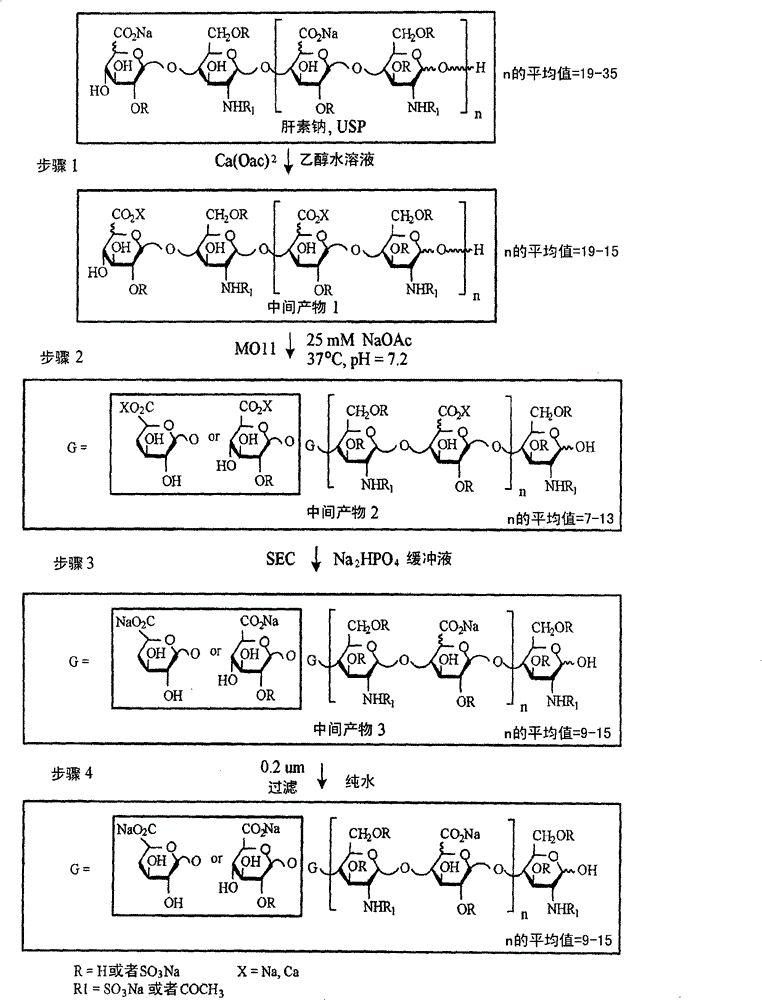
[0464] figure 1A schematic flow chart for the production of M118-REH is shown. Briefly, in the first step of the protocol, commercially available unfractionated heparin USP (UFH) was serially precipitated using calcium acetate in ethanol in water (3:1 mass ratio calcium acetate / UFH) to extract the low molecular weight Fraction of UFH (also referred to as fraction of substantially fast moving components). The product isolated in Step 1 is referred to as Intermediate 1.
[0465] Step 2 involves digesting intermediate 1 with a modified heparanase III having a histidine to Amino acid residue substitution at position 225 of alanine (MO11). MO11 is cleaved by β-elimination between an N-acetylglucosamine residue and a subsulfated uronic acid to produce a Δ4,5 uronic acid group at the non-reducing end and N-acetylglucosamine at the reducing end chain. When the degradation was complete, the heating was stopped...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap