Pharmaceutical composition comprising aspirin, metformin, and serotonin with non-ionic surfactant
A non-ionic surface and metformin technology, applied in the direction of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., can solve problems such as incurable and limited effectiveness of drug treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
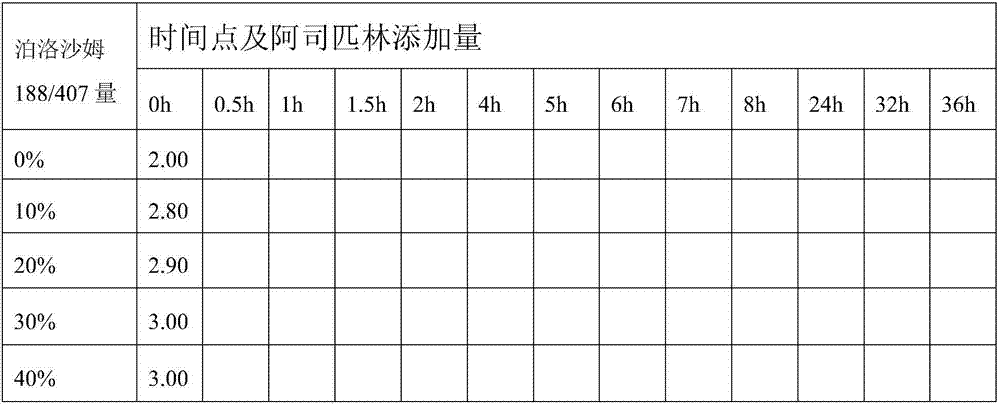
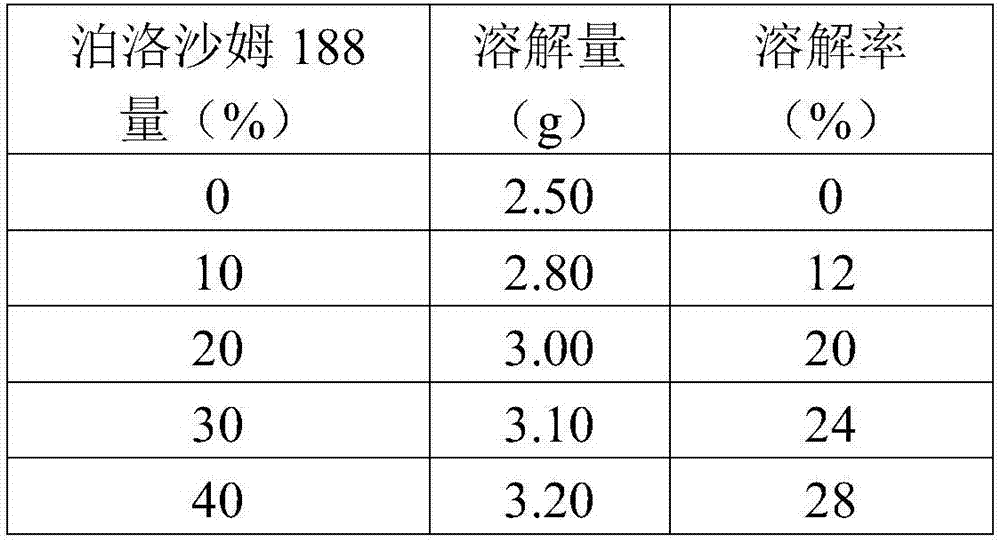
[0091] Solubility test of poloxamer 188 / 407 for aspirin in ethanol
[0092] method
[0093] (1) Prepare 0%, 10%, 20%, 30%, 40% (m / v) poloxamer 188 / 407 absolute ethanol solutions respectively, and put each solution of 10ml into 5 sealed centrifuges tube (mark accordingly on each centrifuge tube).
[0094] (2) According to the pre-test data, add an appropriate amount of aspirin into each centrifuge tube (please refer to Table 1: 0 hour data), and shake to dissolve at room temperature (30°C).
[0095] (3) Observe the centrifuge tube at each specific time interval, as shown in Table 1. When complete dissolution is observed, add 0.1 g of aspirin, then seal and continue shaking. In cases when the drug can no longer be dissolved, the previously dissolved amount of the drug can be recorded as the maximum amount required for dissolution.
[0096] (4) The solubility observation is continued until a certain amount of drug can no longer be dissolved.
[0097] (5) The observation ...
Embodiment 2
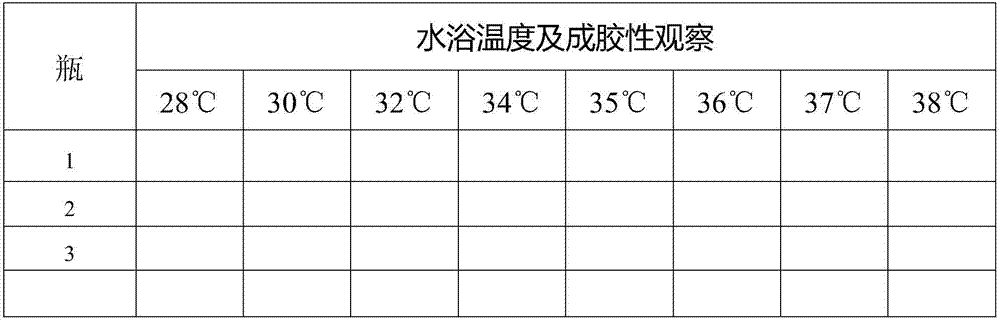
[0110] Gelation temperature measurement test
[0111] method
[0112] (1) Prepare 10 mL of temperature sensitive formulation mixture. The preparation includes equal volumes of Mixture A and Mixture B, as follows:
[0113] Each 1-mL aliquot of mixture A contains: 75 mg of metformin hydrochloride; 5 mg of serotonin-creatinine sulfate complex; 68.75 mg of poloxamer 407; 18.75 mg of poloxamer 188; 0.5 mg of sodium metabisulfite; and water for injection to a total volume of 1 mL. Each 1-mL aliquot of mixture B contained: 200 mg of aspirin; 450 mg of poloxamer 407; 5 mg of tartaric acid; and absolute ethanol added to a total volume of 1 mL.
[0114] (2) The drug mixture is then added to a 25-mL serum bottle (with stir bar).
[0115] (3) Put the serum bottle in a water bath at 28° C. for 15 minutes.
[0116] (4) When stirring is started, it can be observed whether the stirring bar can rotate. When the stirring bar stops rotating, the previous gel temperature can be recorded...
Embodiment 3
[0127] Dissolution release test
[0128] method
[0129] (1) Prepare 10 mL of formulation solution. Prepare this formulation solution according to the following instructions: Prepare a 10-mL solution containing equal volumes of Mixture A and Mixture B. Each 1-mL aliquot of mixture A contains: 75 mg of metformin hydrochloride; 5 mg of serotonin-creatinine sulfate complex; 68.75 mg of poloxamer 407; 18.75 mg of poloxamer 188; 0.5 mg of sodium metabisulfite; and water for injection to a total volume of 1 mL. Each 1-mL aliquot of mixture B contained: 200 mg of aspirin; 450 mg of poloxamer 407; 5 mg of tartaric acid; and absolute ethanol added to a total volume of 1 mL.
[0130] (2) Then slowly add the solution into 15-mL centrifuge tubes (3 mL per tube), to ensure that no solution gets stuck on the wall of the test tube, and at the same time keep the liquid level of each tube at the same height.
[0131] (3) Place the test tube in a 37°C cell culture incubator for 30 minut...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap