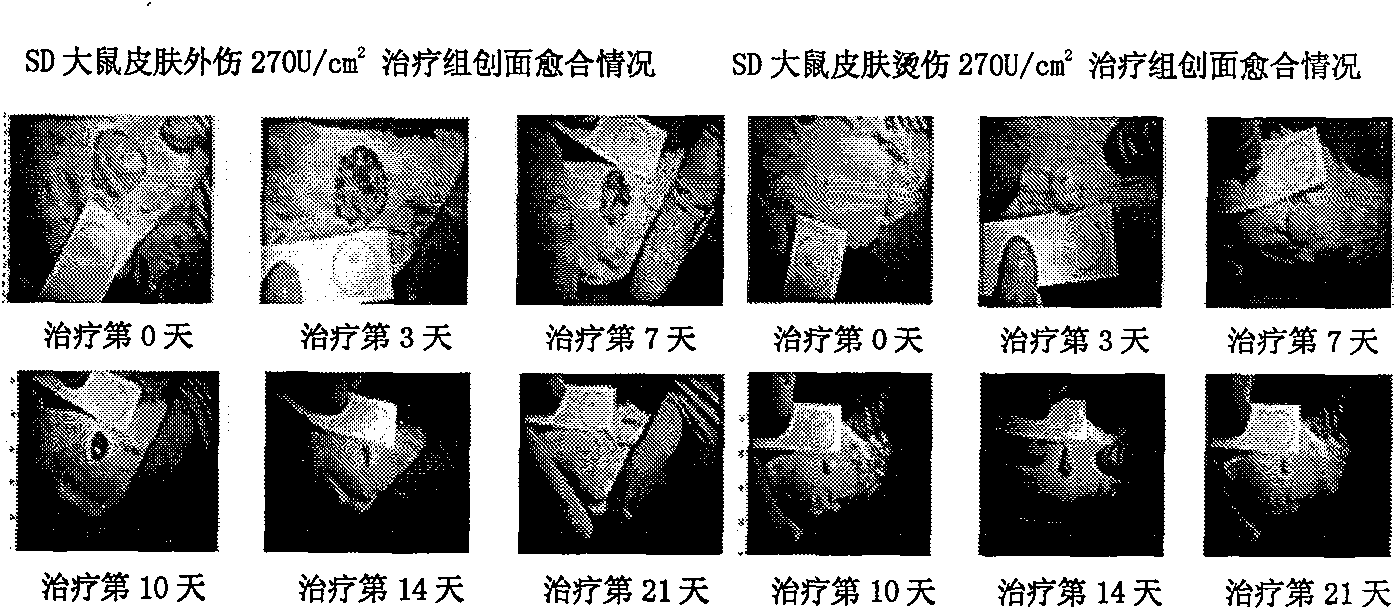
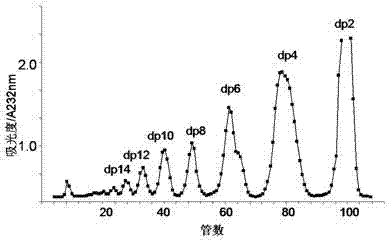
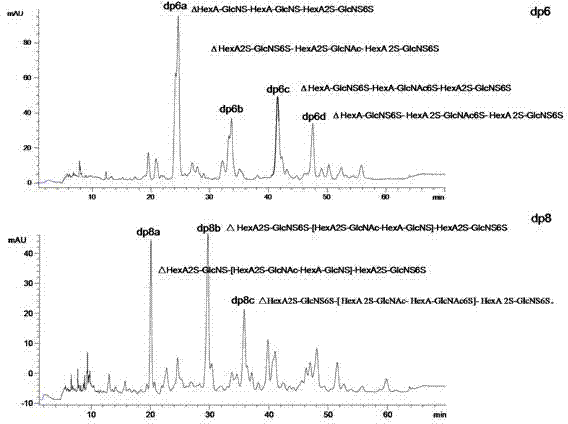
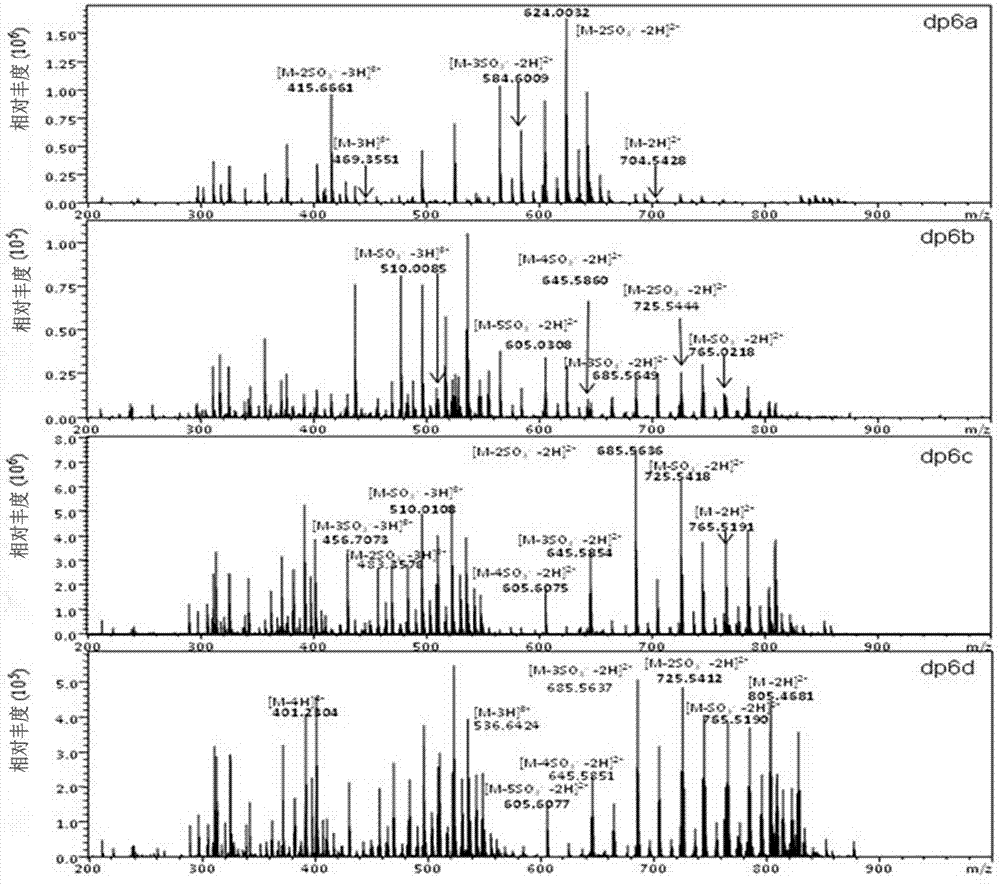
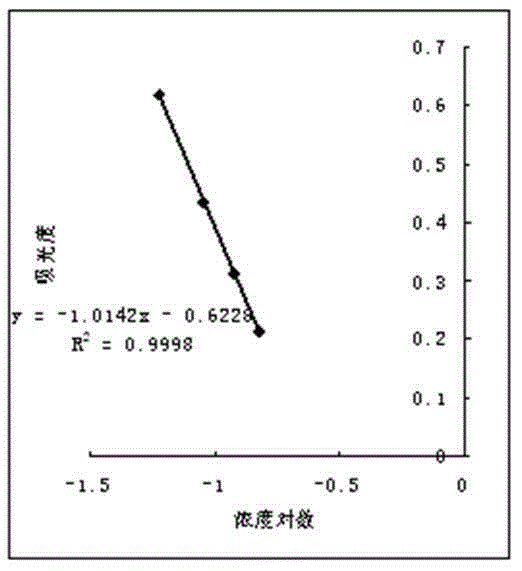
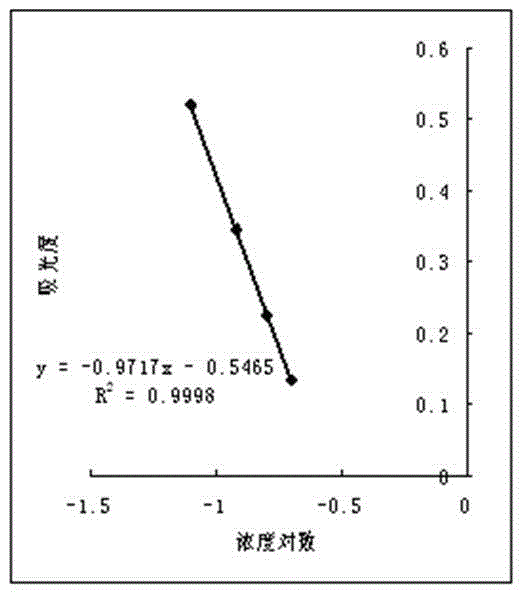
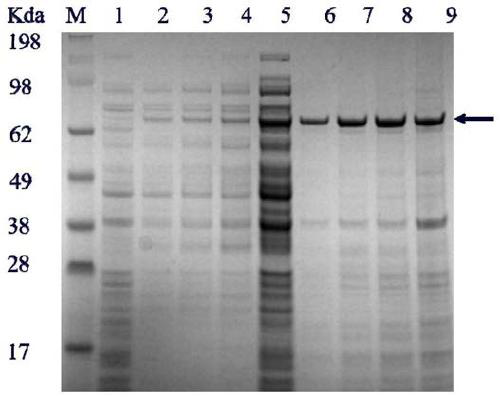
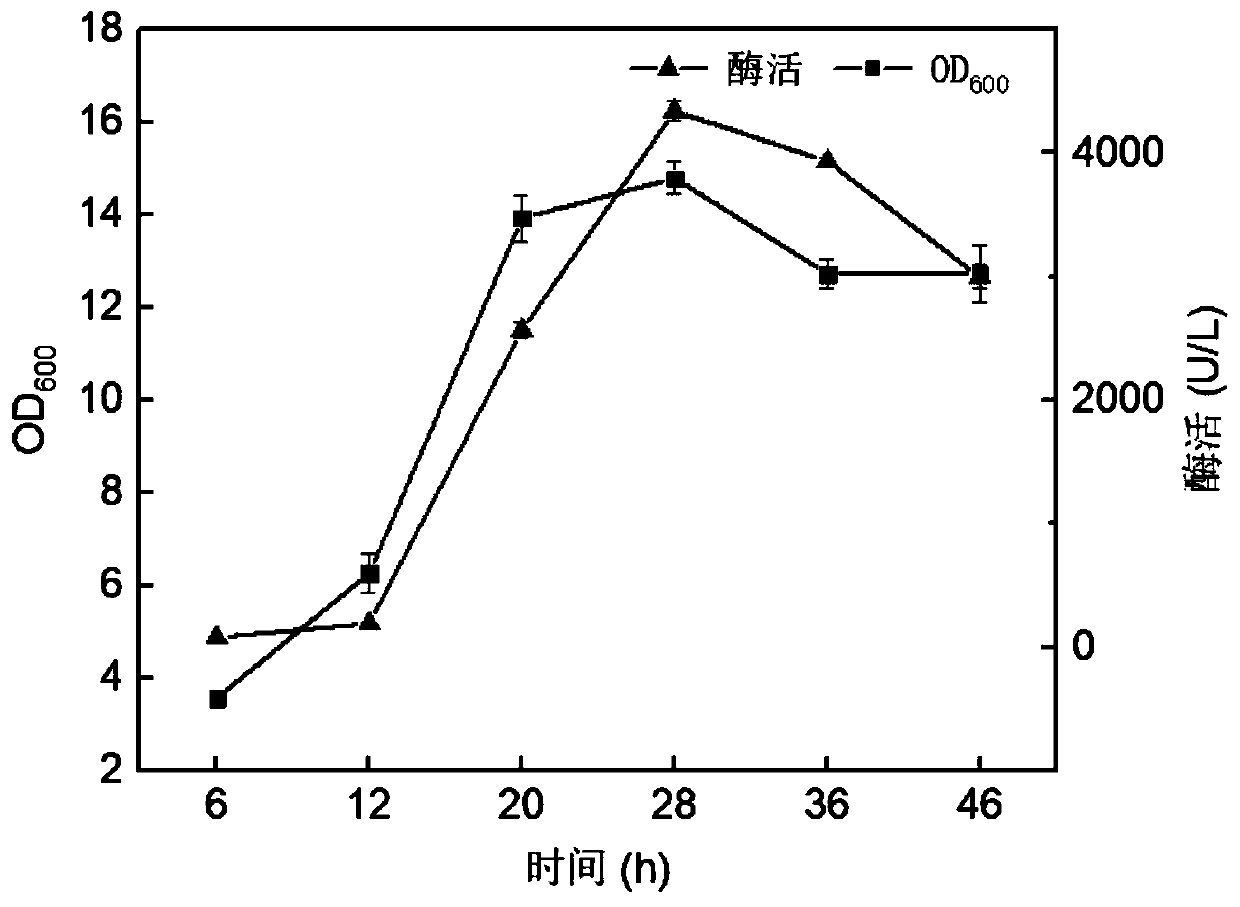
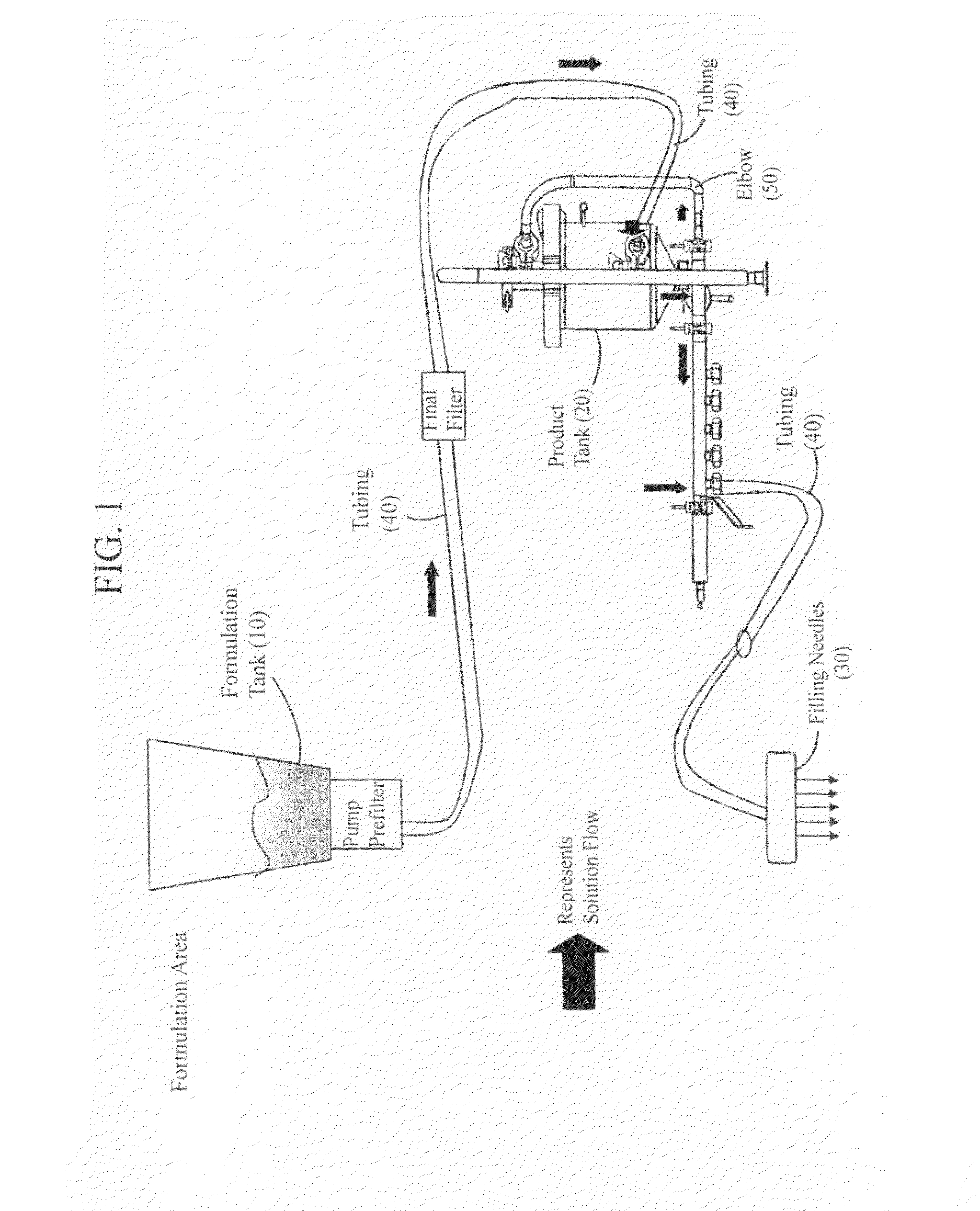
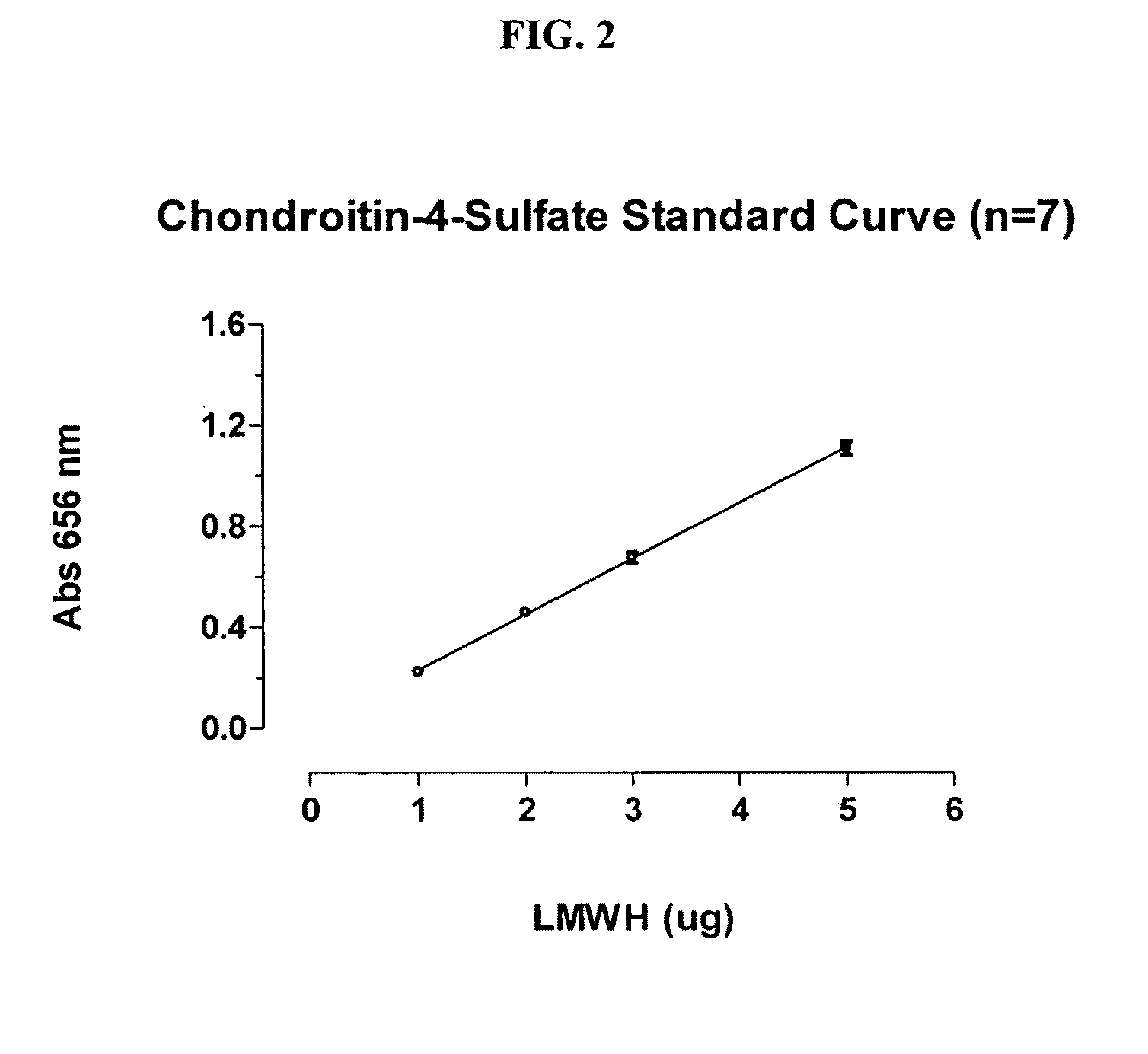
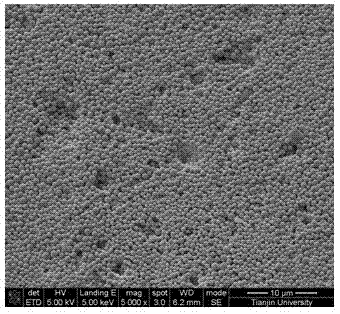
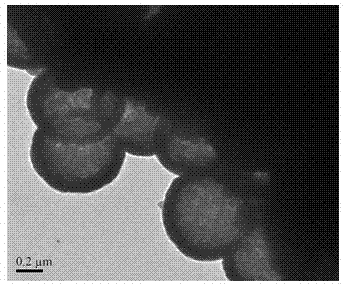
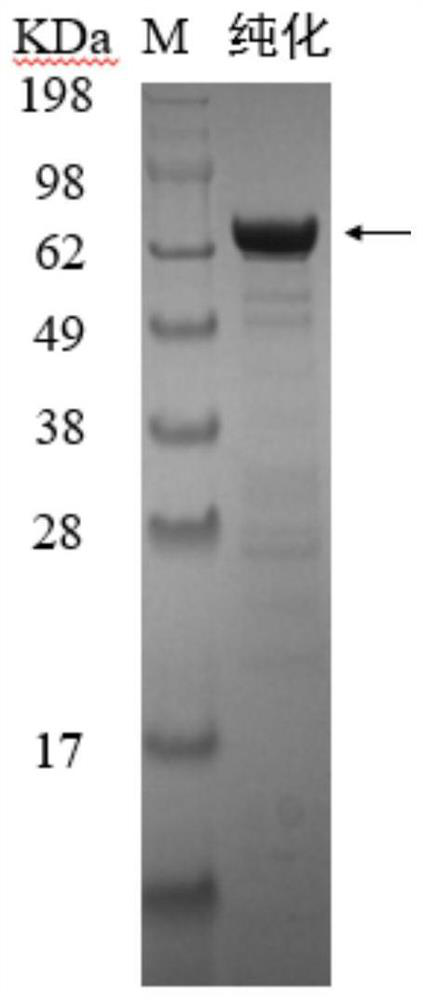
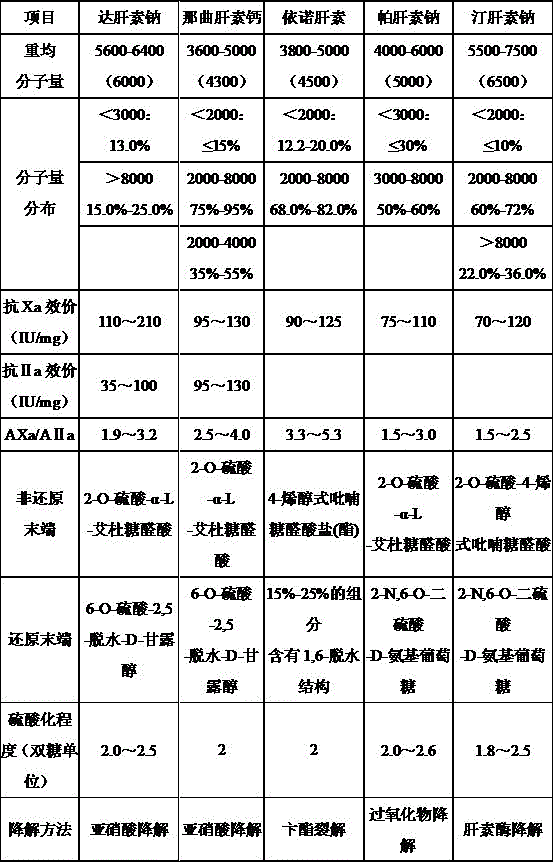
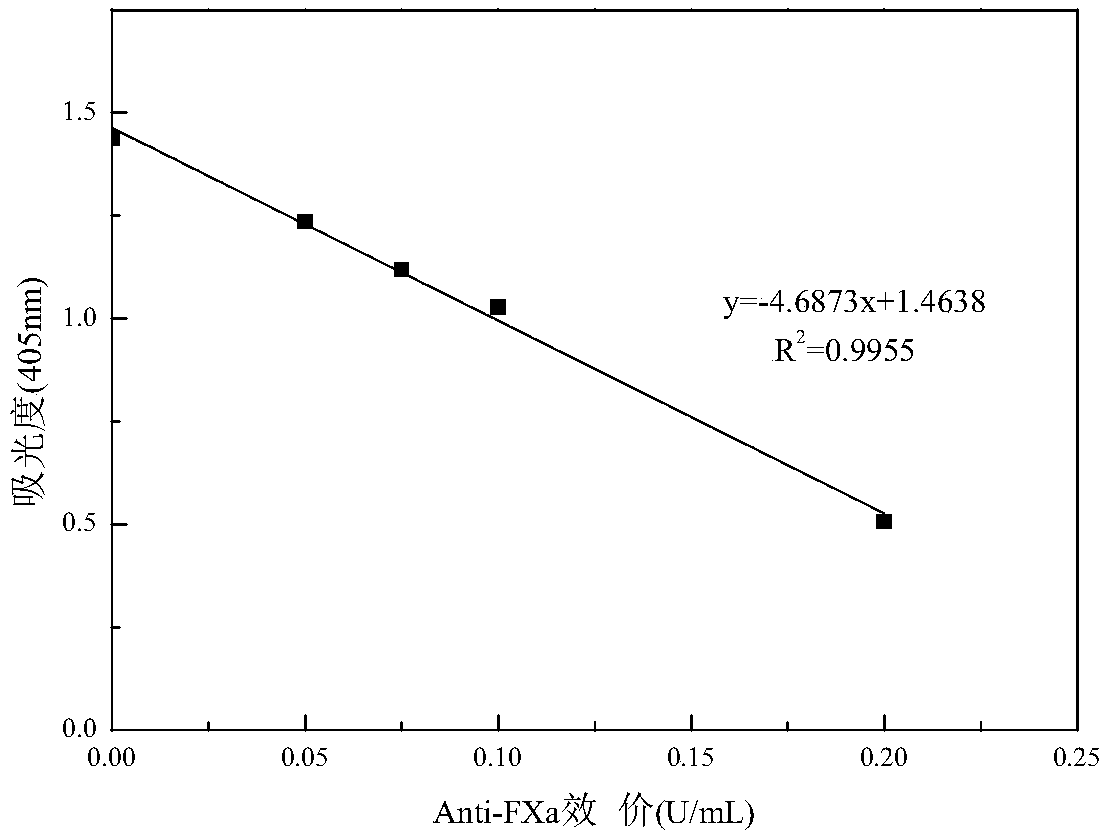
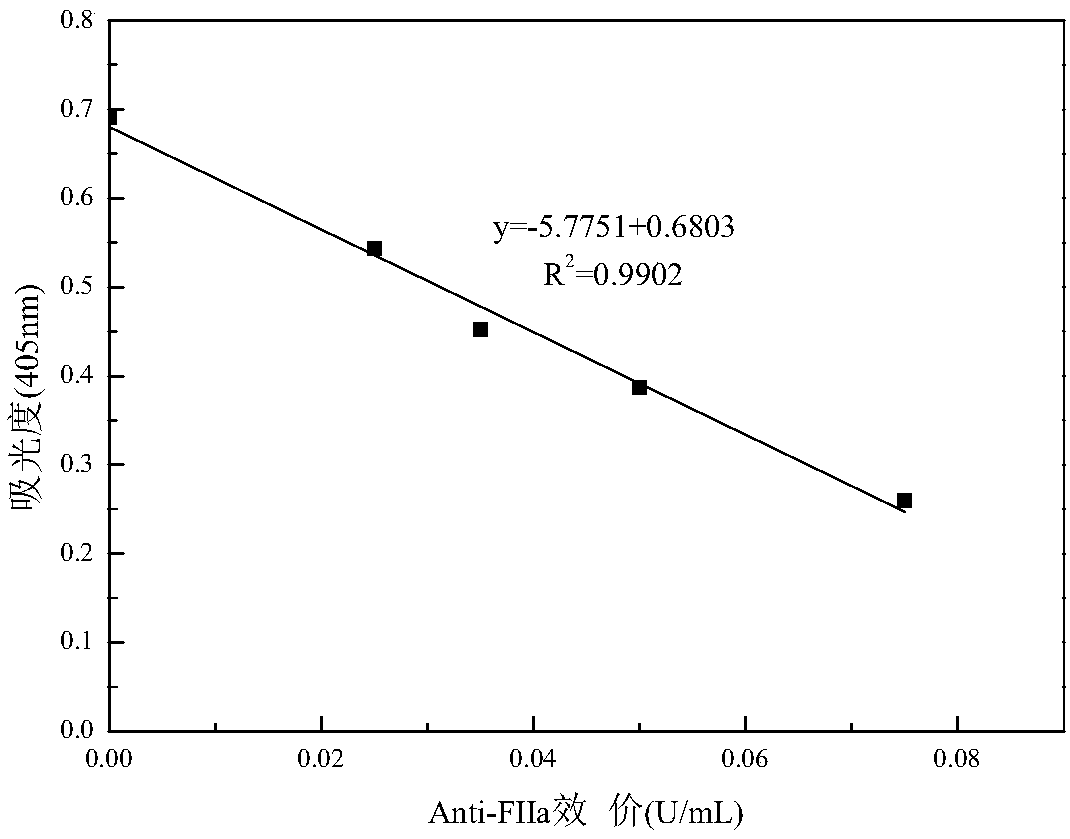
Patents
Literature
56 results about "Heparin.low molecular weight" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Whereas standard heparin has a molecular weight of 5,000 to 30,000 daltons, low-molecular-weight heparin ranges from 1,000 to 10,000 daltons, resulting in properties that are distinct from those of traditional heparin.
Process for preparing and purifying ultra low molecular weight heparin
InactiveCN102040671ASolve the separation problemNo pollution in the processUltrafiltrationHollow fibreLiquid temperature
The invention discloses a process for preparing and purifying ultra low molecular weight heparin sodium (calcium), which comprises the following steps of: reacting heparin with organic quaternary ammonium salt to generate heparin quaternary ammonium salt, performing nucleophilic substitution to generate heparin benzyl ester, and degrading under the alkaline condition to obtain a low molecular weight heparin fragment; and separating and purifying by an inorganic ceramic ultrafiltration and hollow fiber ultrafiltration combined method to obtain the ultra low molecular weight heparin sodium (calcium) of which the molecular weight distribution is 2,000 to 2,500D and the average molecular weight is 2,200D. The low molecular weight heparin fragment is obtained by controlling reaction conditions in the esterification process and the degradation time of ester hydrolysis; a ceramic membrane and a hollow fiber ultrafiltration membrane are combined to separate and purify the heparin fragment; and by selecting the pore diameter of the ceramic membrane, operating pressure, feed liquid temperature, and the molecular weight cutoff of the hollow ceramic membrane, the heparin fragment with a reasonable molecular weight distribution range is effectively separated.
Owner:BEIJING GUANHONG TECH
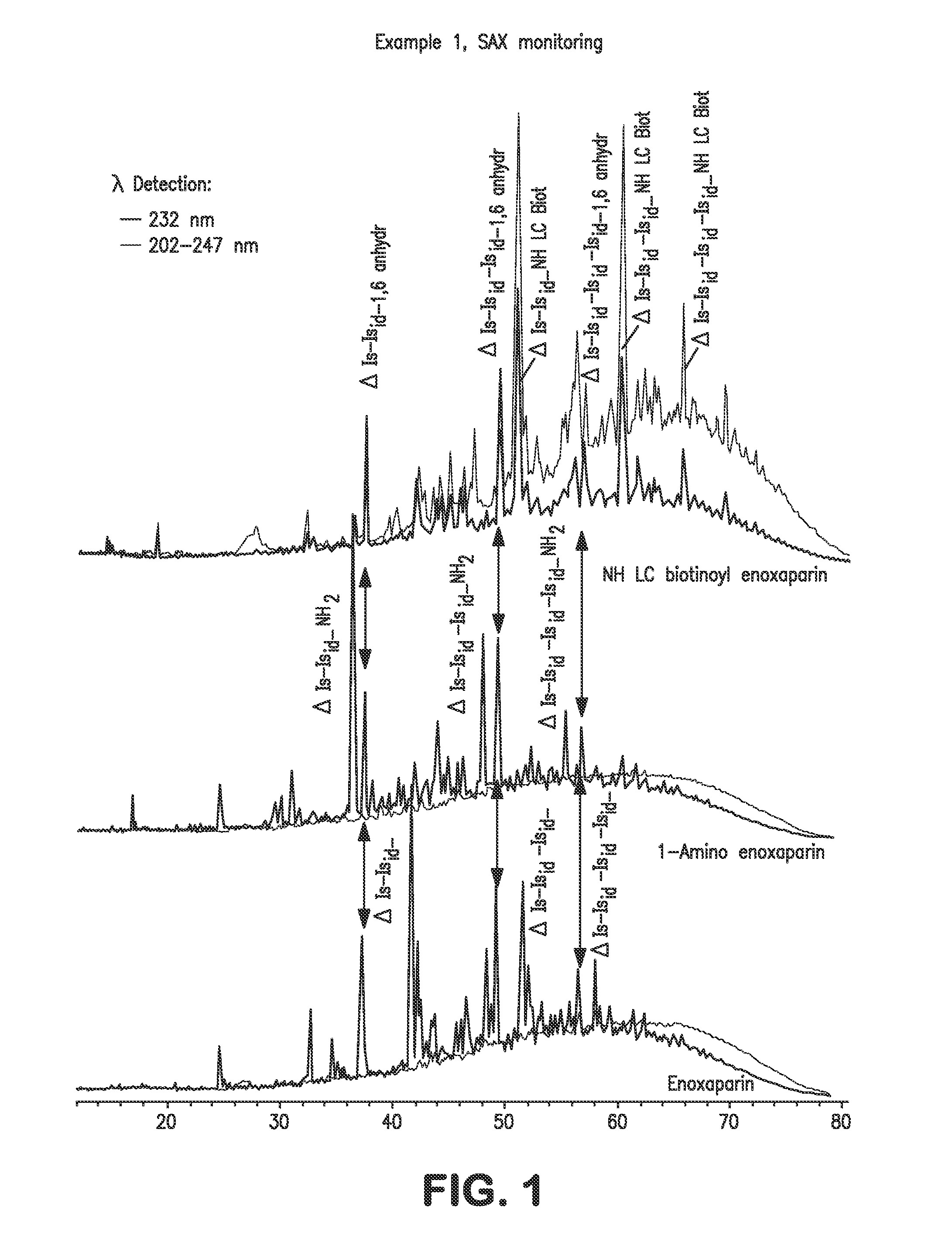
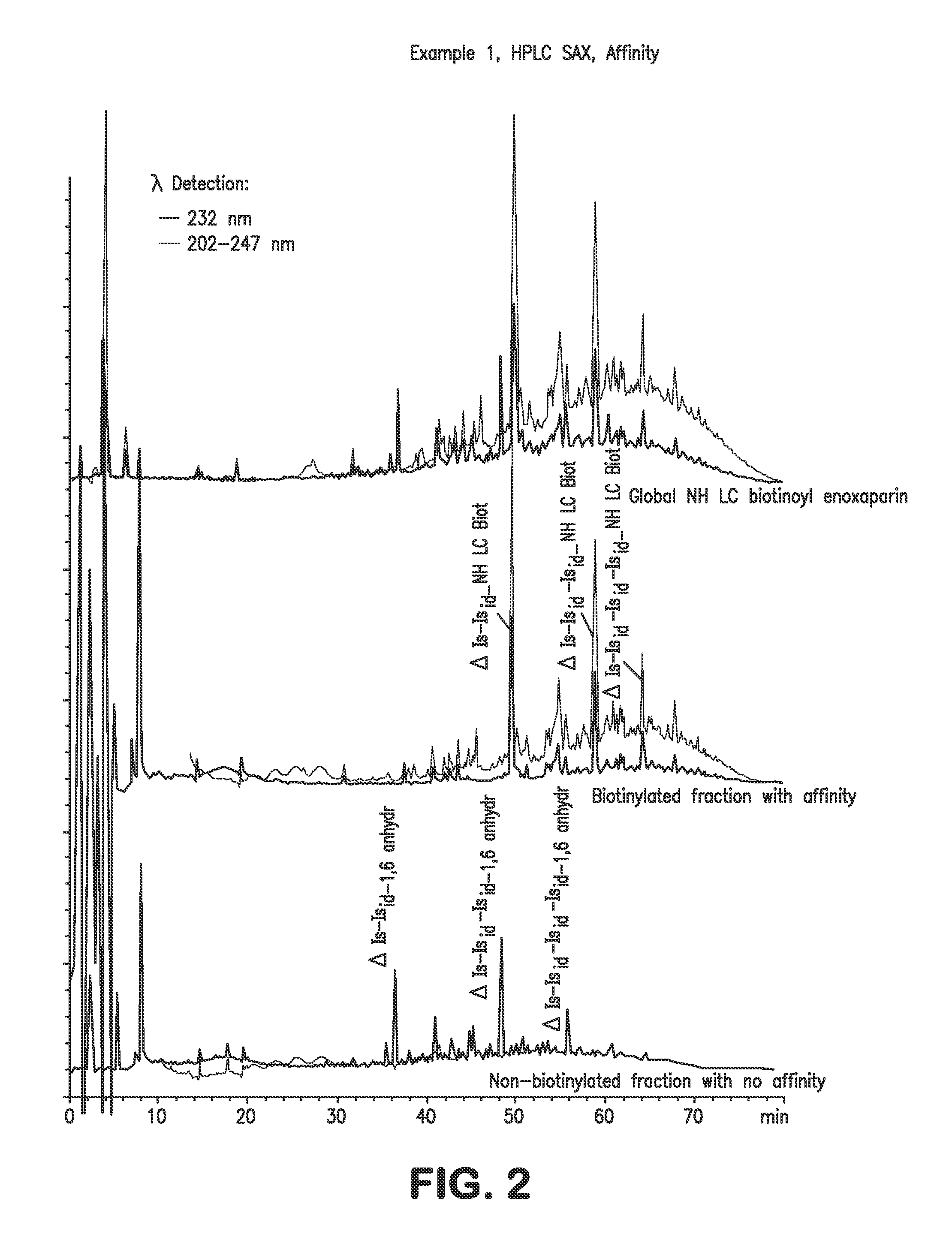
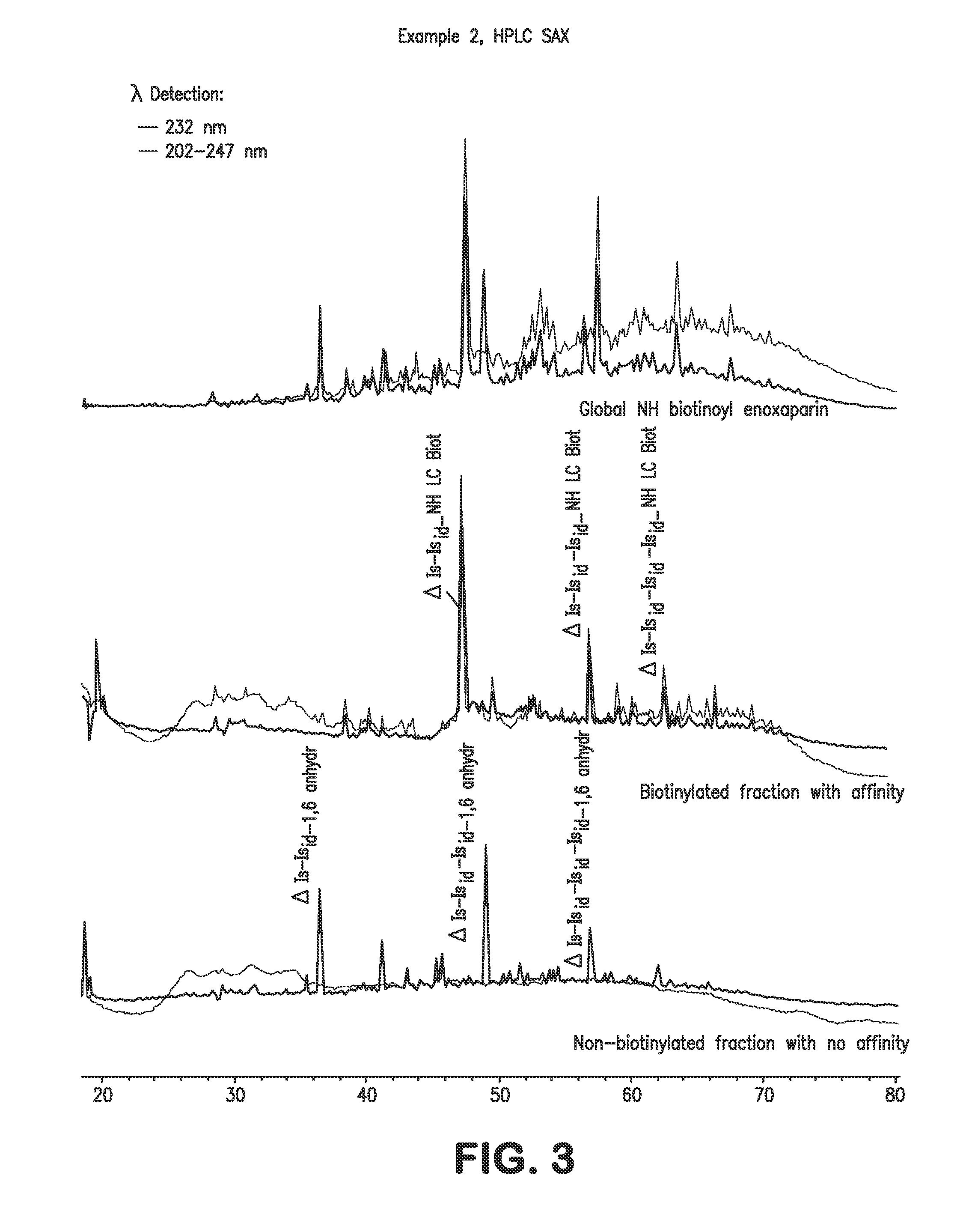
Low molecular weight heparins including at least one covalent bond with biotin or a biotin derivative, method for making same and use thereof
InactiveUS20100081629A1Fast neutralizationReduce riskOrganic active ingredientsNervous disorderBiotinPolysaccharide
The invention relates to biotinylated low molecular weight heparins comprising constituent polysaccharides having at their reducing ends at least one covalent bond with biotin or a biotin derivative, and also to the process for preparing them, to pharmaceutical compositions containing them and to their therapeutic use.
Owner:SANOFI SA
Preparation of gel containing fibroblast growth factor-1 modified body and application thereof for treating diabetic foot
ActiveCN103083226ANon-irritatingEasy dischargePeptide/protein ingredientsMetabolism disorderGlycerolDiabetic foot
The invention provides a preparation method of a gel containing a fibroblast growth factor-1 modified body and an application thereof for treating chronic skin ulcer caused by diabetic foot. The gel disclosed by the invention comprises the following components: 20000-80000 IU of recombinant human fibroblast growth factor-1 modified body (FGF-1135) protein, 10 pg to 40 mu g of low molecular weight heparin sodium, 0.2-1.0 g of glycerol, 50-150 mg of a protein protectant, and 0.03-0.05g of a gel substrate, wherein the pH value is adjusted; and water for injection is added to be 10 ml. Compared with the original sequence, the fibroblast growth factor-1 modified body used in the invention is more steady; after being prepared into the gel, the fibroblast growth factor-1 modified body is more convenient for clinical application; the better stability and slow-release effects can be achieved; the bioavailability of protein is increased; the gel is more beneficial to treating diabetic foot; and the gel can be also used for treating burning, scalding and other chronic and refractory ulcers.
Owner:安徽鑫华坤生物工程有限公司
Preparation method of oligosaccharide containing N-acetylated structure heparin
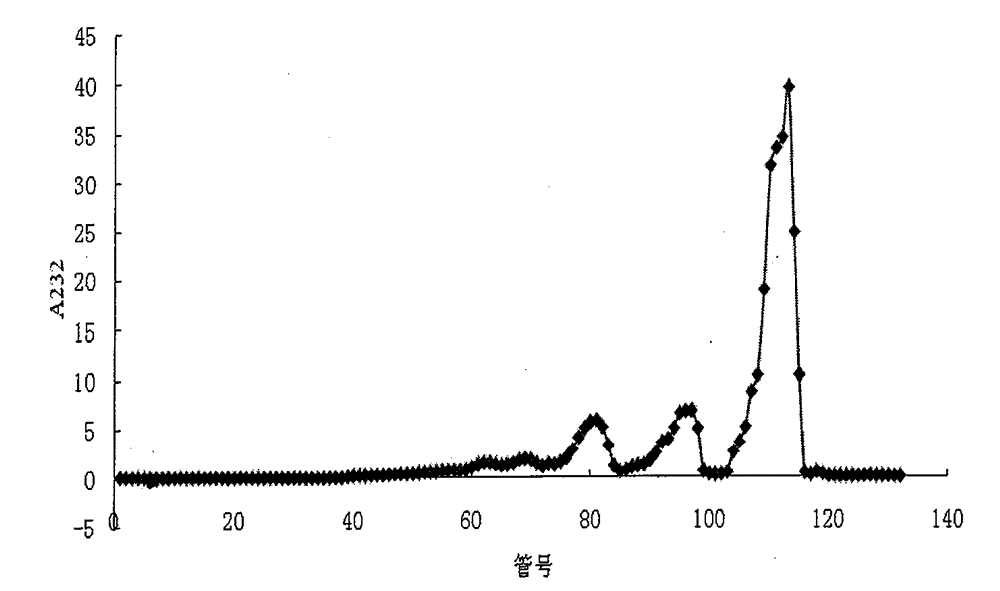
The invention relates to a preparation and purification method of a carbohydrate library containing N-acetylated heparin. The method comprises the following steps: enriching oligosaccharide containing an N-acetylated structure through deep enzymolysis of low molecular weight heparin with heparinase I, then, preparing a series of heparin oligosaccharide crude samples ranging from disaccharide to tetradecasaccharide by Bio-Gel P10 gel chromatography, further separating the crude samples by means of strong anion high performance liquid chromatography and other methods, and respectively purifying the crude samples to obtain four hexasaccharide segments and three octasaccharide fragments; analyzing the disaccharide constituent of each purified oligosaccharide by compound enzymolysis with heparinase I, heparinase II and heparinase III and strong anion chromatography, and primarily deducing sequence structures of the four hexasaccharide and the three octasaccharide in combination with heparinase I substrate specificity; and finally, identifying the structure by electrospray ionization-ion trap-time of flight mass spectrometry (ESI-IT-TOF-MS). The preparation method provided by the invention can be used for solving the problem of difficulty in preparation and structure determination of oligosaccharide containing N-acetylated heparin, which makes research on a relationship between a special structure and functions of heparin / heparan sulfate developed further.
Owner:FUZHOU UNIV
Method for preparing low molecular weight heparin with high activity
InactiveCN101591401AHigh coagulation activityLow costBlood disorderExtracellular fluid disorderHeparin biosynthesisSulfate
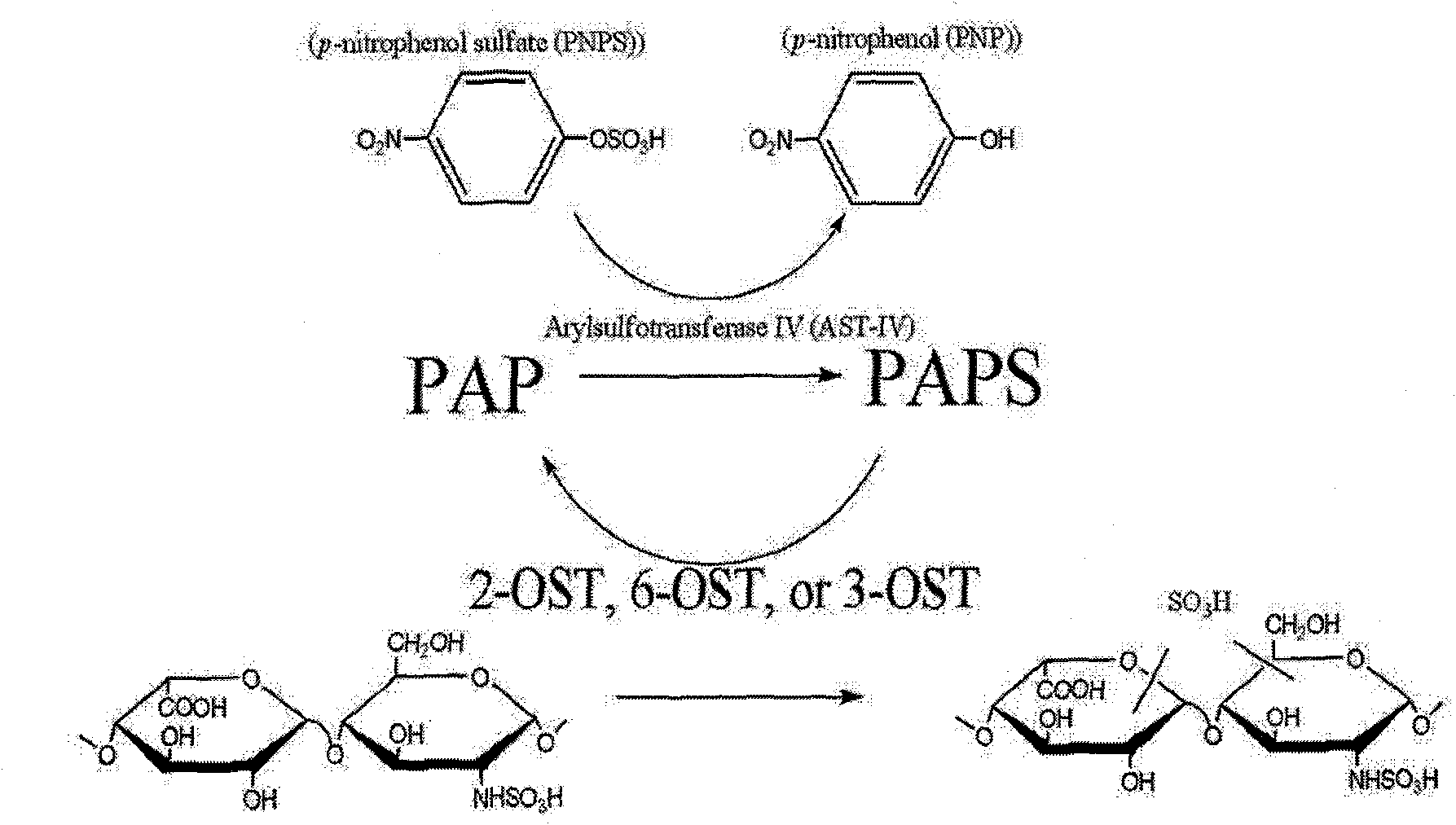
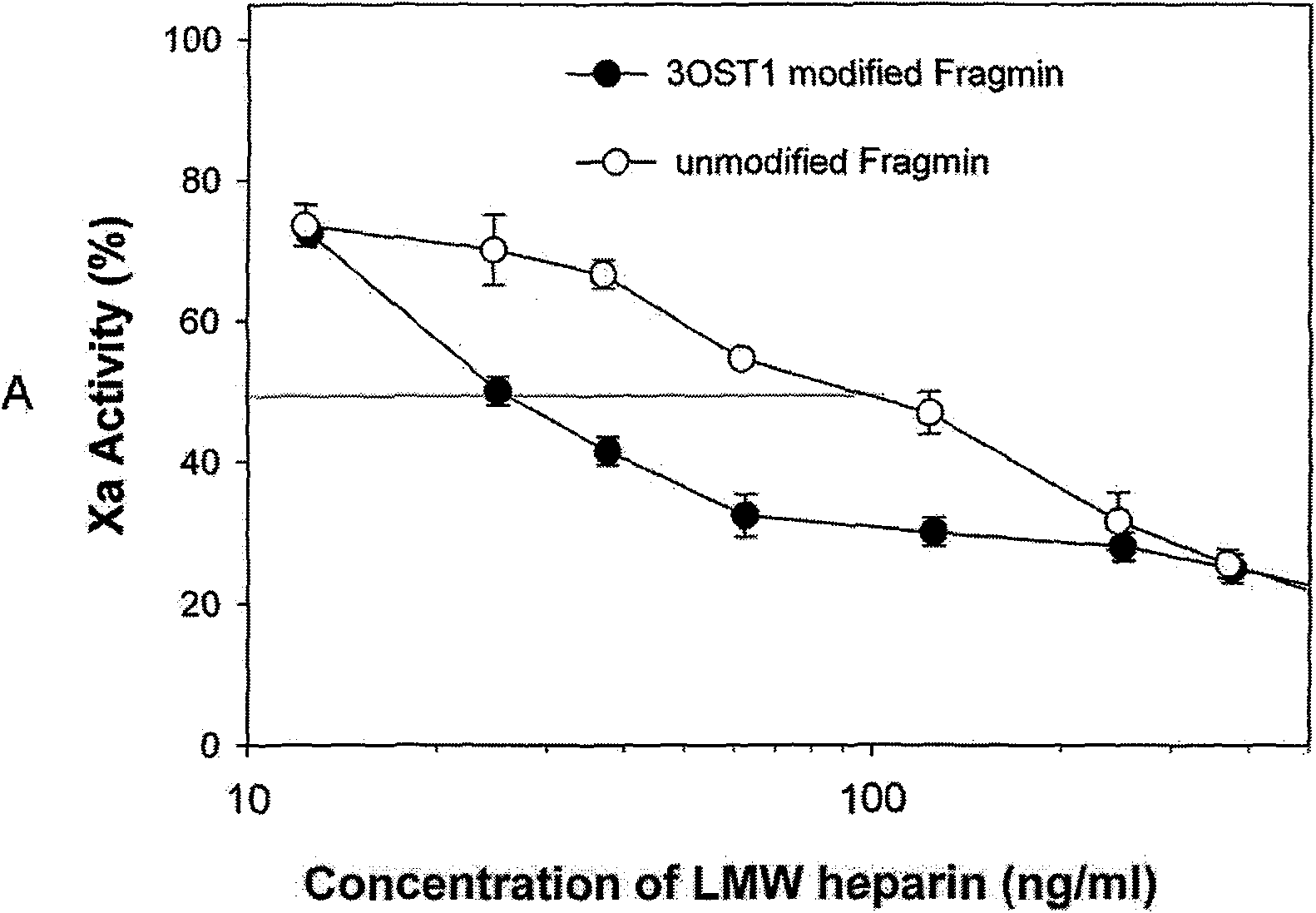
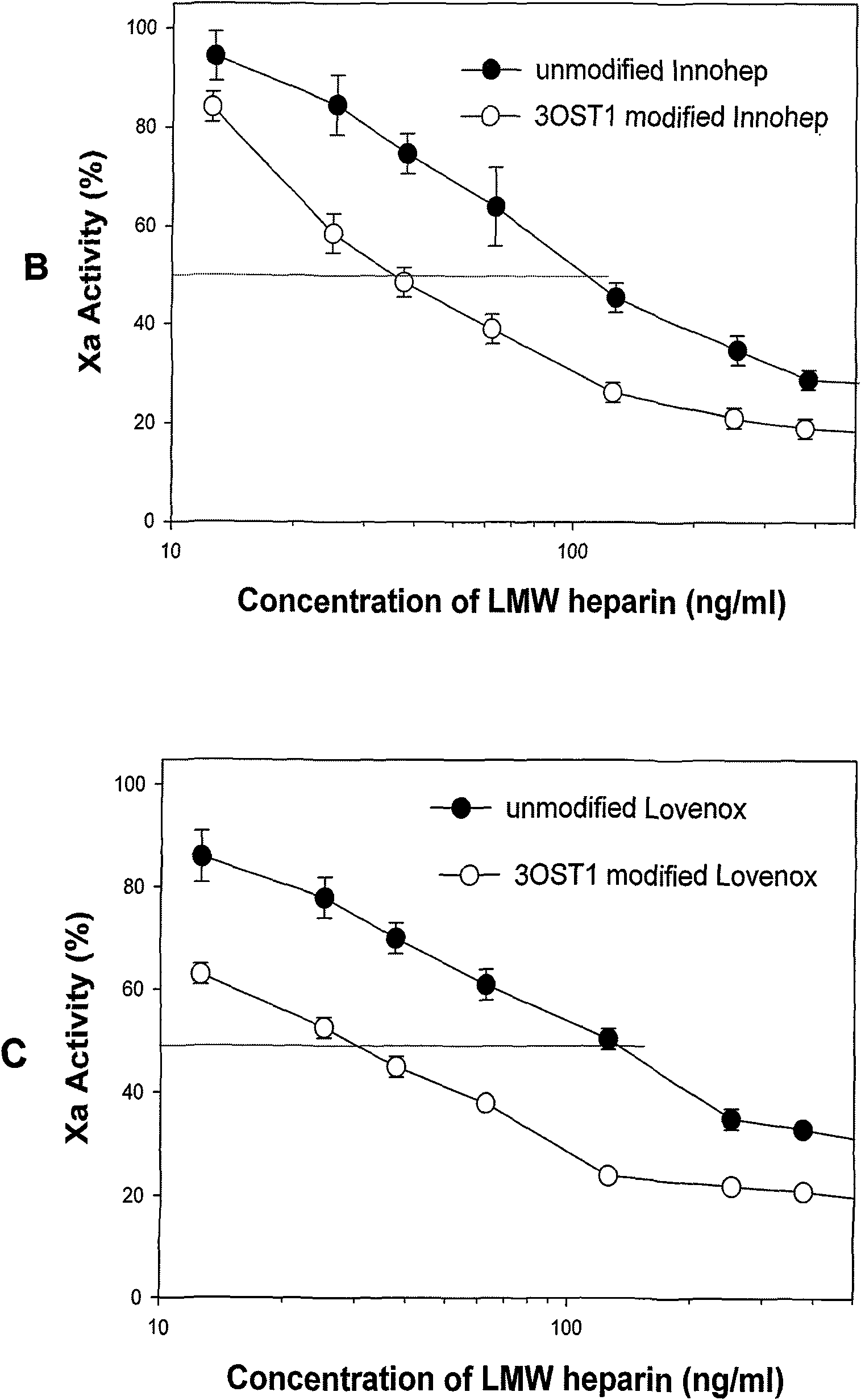
The invention discloses a method for preparing low molecular weight heparin with high activity, which is characterized in that the prior low molecular weight heparin with low anticoagulant activity is used as a substrate, a PAPS (3'-phosphoadenosine-5'-phosphoric acid sulfuric acid) regeneration system is adopted, and a heparin biosynthetic enzyme 3-O-sulfate-transferase is used for modifying the substrate to obtain the low molecular weight heparin with high anticoagulant activity; and the PAPS regeneration system can use extremely cheap PNPS (p-nitryl potassium phenolsulfonate) as an enzyme-modified reactive sulfate donor to further save the cost and make the industrialization possible. The method adopts the 3-O-sulfate-transferase with high enzyme activity to combine with the PAPS regeneration system, performs selective modification on the low molecular weight heparin, and increases the number of anticoagulant activity centers so as to greatly improve the anti-thrombosis activity of the heparin. The method provides a path for the enzymatic industrial production of the low molecular weight heparin with high activity.
Owner:JIANGNAN UNIV
Heparin disaccharide mixture and preparation method and application thereof
InactiveCN102864191AComponent separationMicrobiological testing/measurementHigh-performance liquid chromatographyHeparin disaccharide
The invention relates to heparin disaccharide mixture and a preparation method thereof. The disaccharide mixture is used as a standard substance for conducting high performance liquid chromatography (HPLC) analysis for heparin or low-molecular heparin. The invention further relates to the application of the disaccharide mixture.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Method for controlling the production of low molecular weight heparin
This article discloses a production method of low molecular weight heparin or ultra-low molecular weight heparin. This method utilizes two or more heparinase enzymes selected from heparinase I, II and III to degrade heparin to produce low molecular weight heparin or ultra-low molecular weight heparin.
Owner:TSINGHUA UNIV
Low molecular weight heparin composition and uses thereof
ActiveUS8609632B2Improve pharmacokineticsHigh activityAntibacterial agentsOrganic active ingredientsChemistryLow molecular weight heparin
Preparations of low molecular weight heparins (LMWHs) having improved properties, e.g., properties that provide a clinical advantage, are provided herein. Methods of making and using such preparations as well as methods of analyzing starting materials, processing, intermediates and final products in the production of such LMWH preparations are provided.
Owner:MOMENTA PHARMA
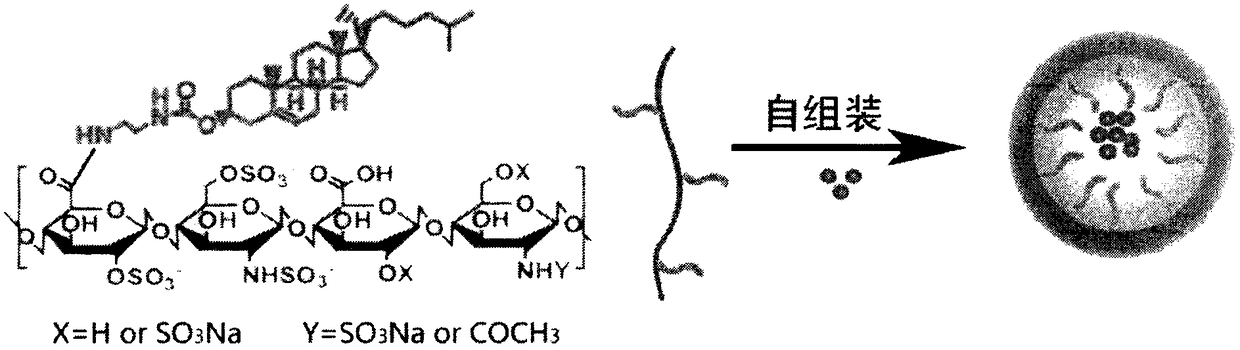
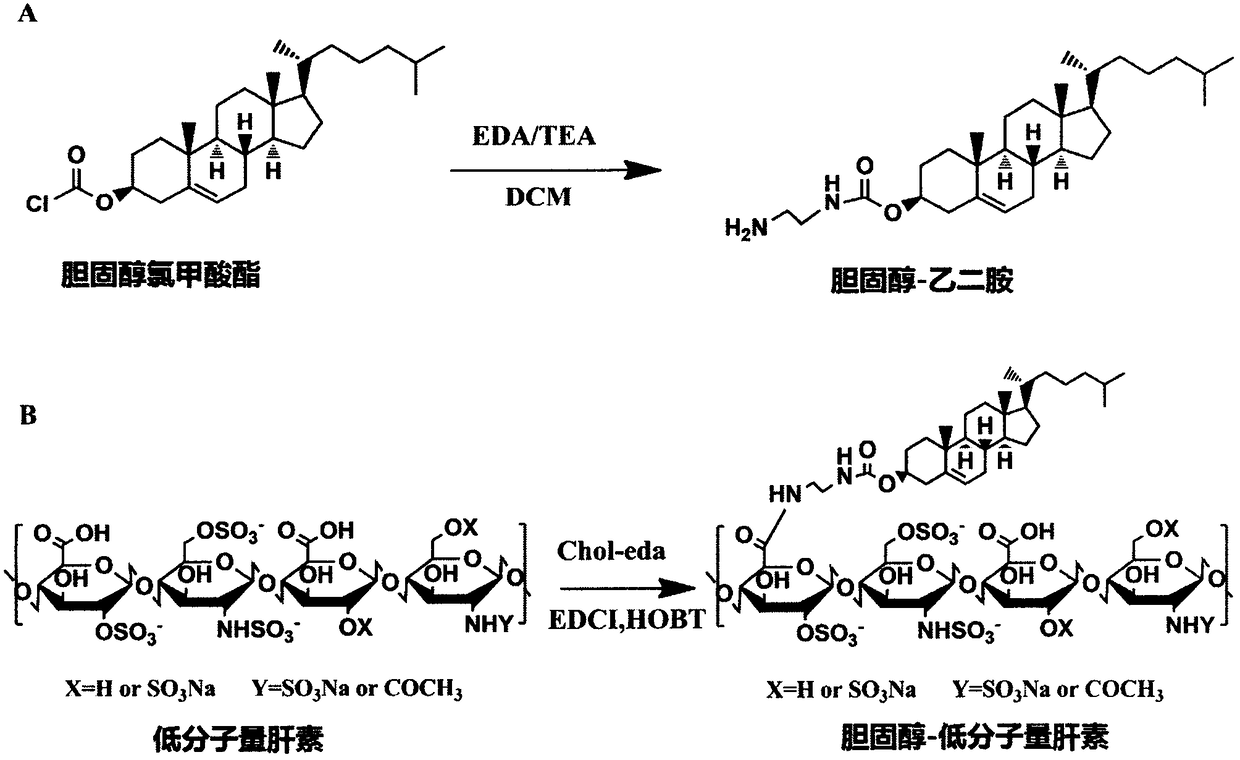
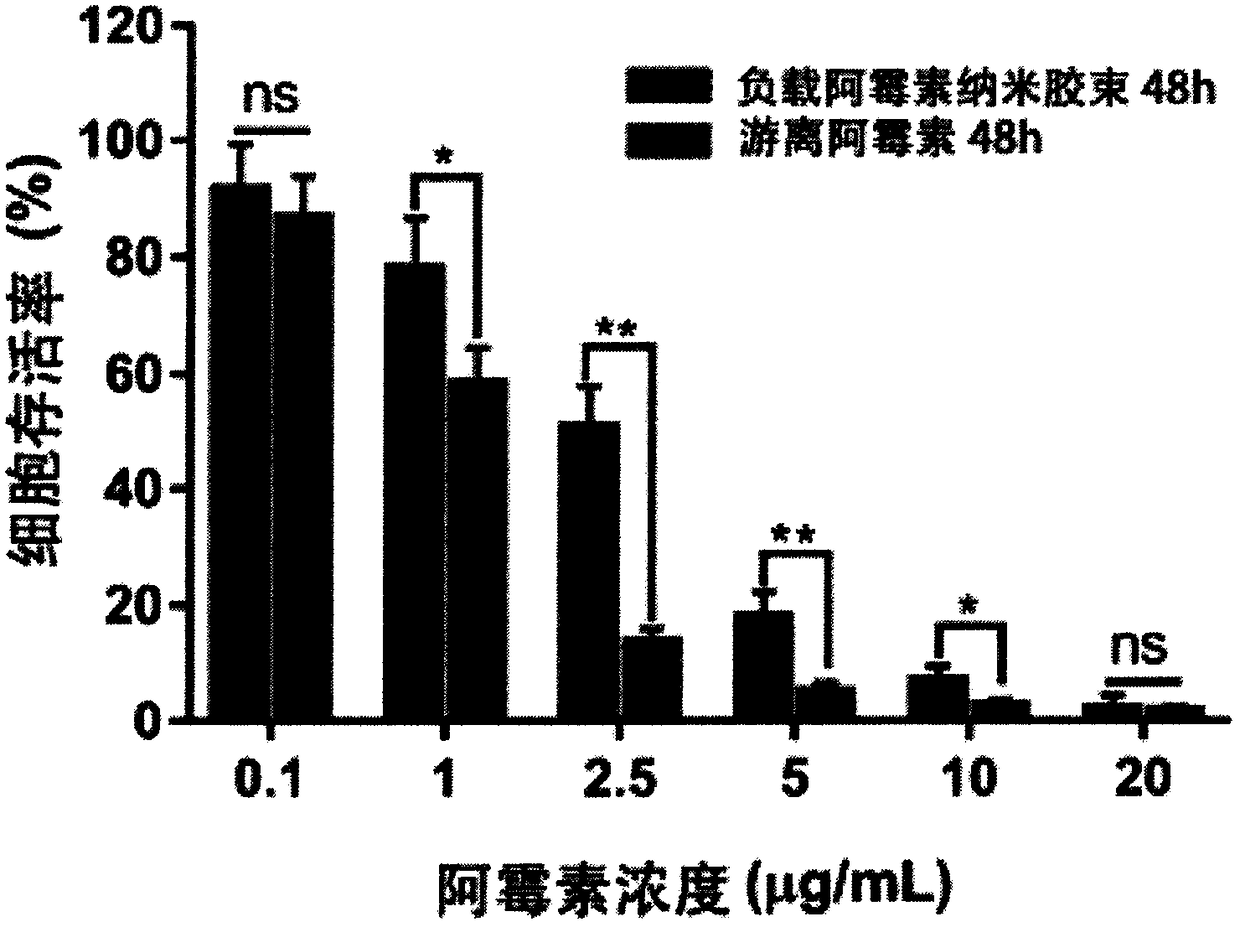
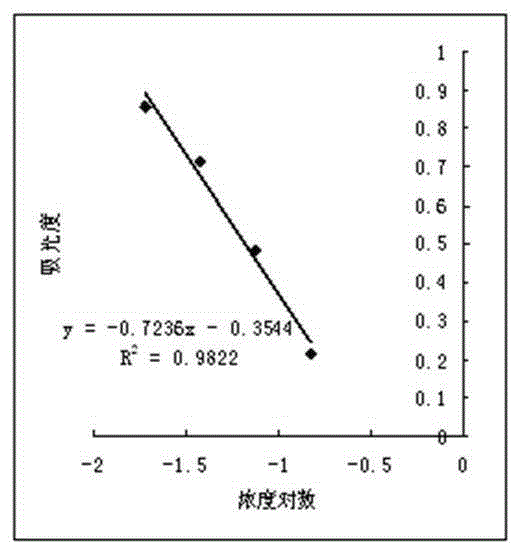
Cholesterol-low molecular weight heparin nano preparation for anti-tumor and anti-metastasis therapy and preparation method thereof
InactiveCN108451906AImprove anti-cancer effectOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolBiocompatibility Testing
The invention relates to a cholesterol-low molecular weight heparin nano preparation loaded with anti-tumor drugs for anti-tumor and anti-metastasis therapy and a preparation method thereof. Specifically, the nano preparation is characterized in that cholesterol with excellent biocompatibility serves as a hydrophobic fragment and a hydrophobic drug reservoir for encapsulation of an anti-tumor drug, hydrophilic low molecular weight heparin with anti-metastasis activity serves as a shell so as to prepare the novel nano preparation for preventing postoperative recurrence, and therefore tumor cells can be killed, and meanwhile, the effect of inhibiting tumor cell metastasis can be achieved. The nano preparation has the advantages that the drug loading capacity is within 5-20wt%, the prepared nano preparation has an injectable property, administration is realized through tail intravenous injection, and therefore the anti-tumor effect of the drug can be improved, and meanwhile, the anti-metastasis activity of the low molecular weight heparin can be exerted.
Owner:CHINA PHARM UNIV
Method for detecting potency of heparin or heparin with low molecular weight through a coagulometer
InactiveCN105203479AReduce experimental errorImprove experimental accuracyPreparing sample for investigationColor/spectral properties measurementsMedicinal chemistryReagent
The invention relates to a detection method for detecting the anti-Xa potency of heparin (salt) or heparin (salt) with the low molecular weight through a coagulometer. The method comprises the following steps of 1 standard serial solution preparing; 2 standard curve drawing; 3 sample processing and detecting; 4 calculating. According to the method, four kinds of standard solution concentrations are achieved through automatic diluting performed with the coagulometer, personal errors are few, all the operation steps are automatically completed, the man-made influences of the time, the temperature and stirring are completely eradicated, and the experiment precision is high; reaction of the used reagents is changed from semimicro reaction to micro reaction, and therefore the expensive reagents are saved; a linear equation method is adopted and is intuitional.
Owner:山东万邦赛诺康生化制药股份有限公司
Heparin lyase mutant and recombinant expression method thereof
ActiveCN111471669AHigh catalytic efficiencyImprove thermal stabilityAntibody mimetics/scaffoldsFermentationHeterologousLyase
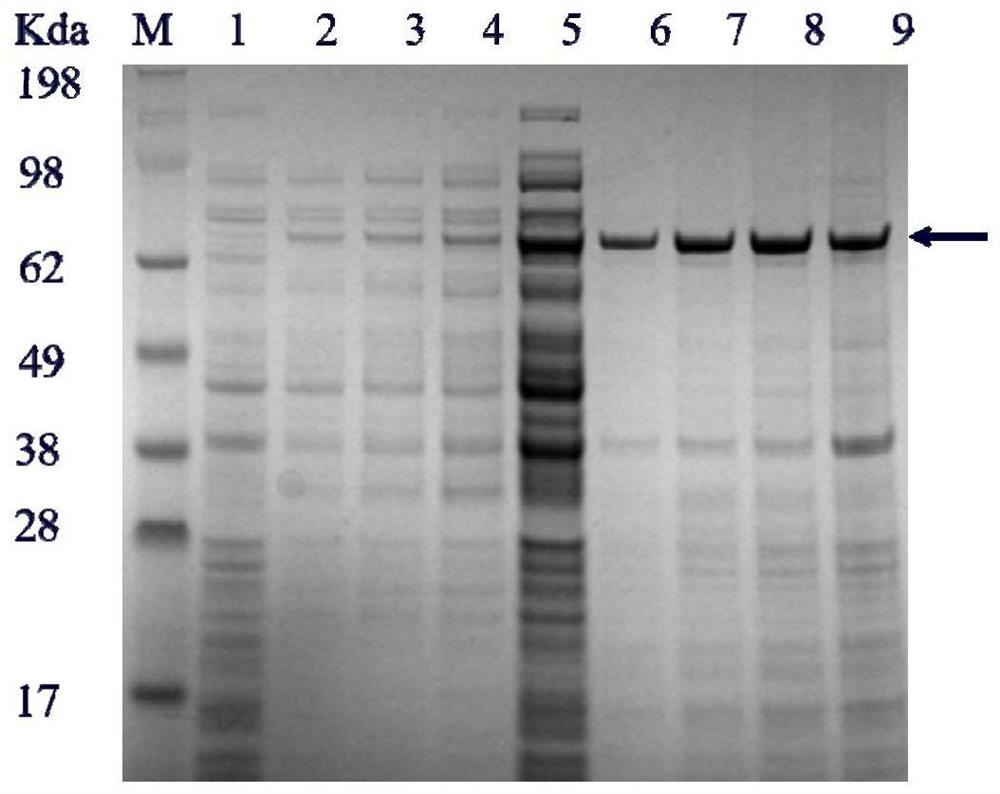
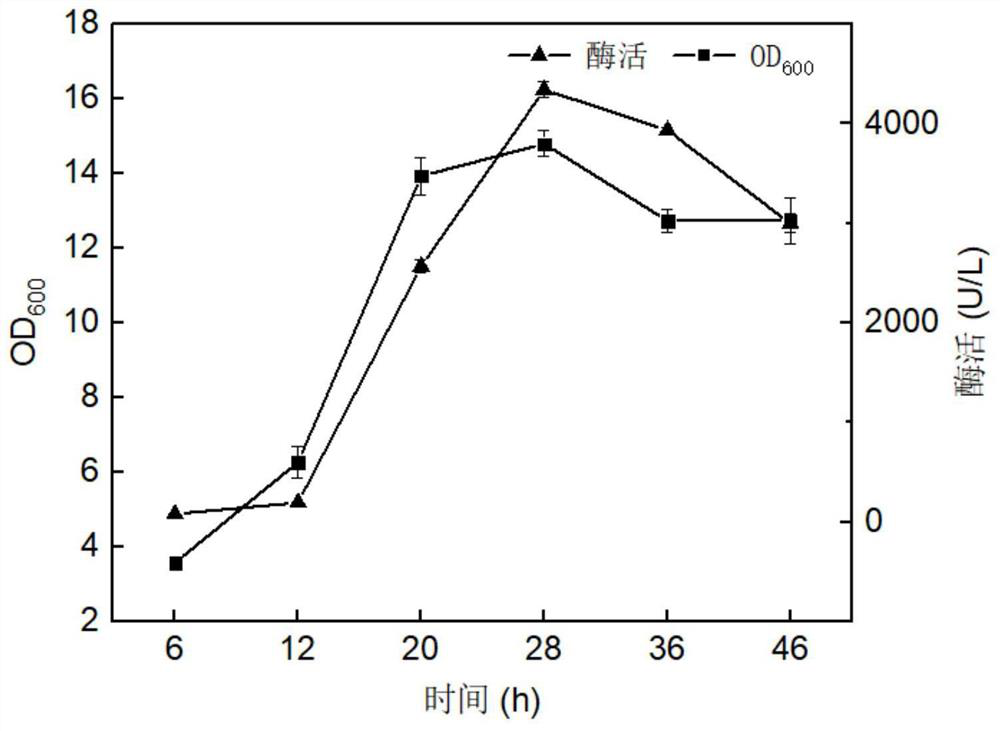
The invention discloses a heparin lyase mutant and a recombinant expression method thereof, and belongs to the technical field of bioengineering. The heparin lyase III provided by the invention has higher catalytic efficiency and thermal stability, the catalytic efficiency of the mutant S264F / Y490K / D321N is improved by 1.68 times compared with that of an original strain, and preparation of low-molecular-weight heparin is facilitated. Heparin lyase III from bacteroides thetaiotaomicron is subjected to heterologous recombination expression, and the intracellular enzyme activity can reach 4000 U / L or above.
Owner:NANJING HANXIN PHARMA TECH CO LTD +1
Application of heparinase II to preparation of LMWH (low-molecular-weight heparin) by depolymerization of heparosan
The invention provides an application of heparinase II to the preparation of LMWH (low-molecular-weight heparin) by the depolymerization of a heparosan and a method for preparing the LMWH by depolymerizing the heparosan by the recombinant heparinase II. The invention provides a new application of the heparinase II to the depolymerization of the heparosan and a new method for preparing the LMWH by the heparinase. The method has the advantages of mild reaction condition, easy separation of the product and the enzyme and convenience of production, thereby having a significant application prospect.
Owner:ZHEJIANG UNIV OF TECH
Method for measuring the concentration of a glycosaminoglycan anticoagulant
ActiveUS20090042303A1Performed easily and quicklyImprove throughputSamplingElectrostatic cleaningAnticoagulantGlycosaminoglycan
The invention provides an accurate, economical, automatable, high throughput method for the determination of the concentration of glycosaminoglycan anticoagulants, including low molecular weight heparin (LMWH) anticoagulants, in aqueous solutions. A method for cleaning a unit of manufacturing equipment used in the preparation of a LMWH to obtain an acceptable residual concentration of LMWH is further provided.
Owner:ACTIVE BIOMATERIALS
Low-molecular-weight heparin affinity purification medium and method for purifying low-molecular-weight heparin
InactiveCN104190383AEasy to collectEfficient preparationOther chemical processesAlkali metal oxides/hydroxidesCross-linkPurification methods
The invention provides a low-molecular-weight heparin affinity purification medium, an affinity purification polyethylene imine dextran microsphere formed by the medium and a low-molecular-weight heparin affinity purification method, belonging to the field of biological medicine. The low-molecular-weight heparin affinity purification medium comprises three components: polyethylene imine, cross-linking substances and a microsphere structure, wherein the cross-linking substances comprise a cross-linking agent and a surfactant; the microsphere structure comprises microspheres and a microsphere carrier. The low-molecular-weight heparin can be well collected and other types of heparins are also removed when the low-molecular-weight heparin is purified by the purification medium; the purpose of well separating and purifying is achieved; the low-molecular-weight heparin can be used for quickly and efficiently preparing low-molecular-weight heparin sodium in a large scale.
Owner:NANJING AGRICULTURAL UNIVERSITY
Preparation method of submicron order core/shell structure PLGA (Poly(Lactic-co-Glycolic) microsphere with uniform particle size
ActiveCN103239408AStable and predictable release behaviorDoes not affect surface topographyPharmaceutical non-active ingredientsGranular deliveryMicrospherePoly dl lactide
The invention discloses a preparation method of submicron order core / shell structure poly(lactic-co-glycolic) microsphere with a uniform particle size. The microsphere is of a core / shell structure, the particle size is 400-600nm, and the average shell thickness is 70nm. The preparation process comprises the following steps of: dissolving bovine serum albumin, carboxymethyl chitosan or low molecular weight heparin into a phosphate buffer liquid to be used as solution 1; dissolving PLGA and a emulsifier F127 into dichloromethane to be used as a solution 2; conducting ultrasonic dispersion on the solution 1 and the solution 2 so as to form a primary emulsion; further dropping the primary emulsion into a calcium chloride solution, and conducting ultrasonic dispersion in an ice bath so as to form a composite emulsion; and volatilizing the solvent, dialyzing, centrifugally washing, freezing and drying so as to obtain the PLGA microsphere. The preparation method has the advantages that the preparation process is simple, and meanwhile due to the core / shell structure of the microsphere, medicines can be wrapped into the core, the particle size is uniform, the medicine release action can be predicted, and the microsphere is convenient to be compounded with a tissue engineering bracket.
Owner:天津渤化讯创科技有限公司
Preparation and purification method of heparin hexasaccharides containing N-unsubstituted glucosamine
The invention relates to a preparation and purification method of heparin hexasaccharides containing (i)N(i)-unsubstituted glucosamine (GlcNH3+). The preparation and purification method comprises the following steps: using enzymolysis low molecular weight heparin as the raw material, separating and purifying the heparin sodium hexasaccharide by methods of gel chromatography and ion exchange HPLC; preparing heparin pyridine hexasaccharide by using cation exchange resin and pyridine neutralization process; finally preparing heparin hexasaccharides with different numbers of GlcNH3+ by the (i)N( / i)-sulfate removal method, and separating and purifying the heparin hexasaccharide by the SAX-HPLC method. The preparation and purification method can specifically prepare the heparin oligosaccharides containing different numbers and sequences of GlcNH3+, and provide important oligosaccharides libraries for researching the structure and function of the GlcNH3+ residues in the heparin sulfate and the relationship between the GlcNH3+ residues and diseases.
Owner:FUZHOU UNIV
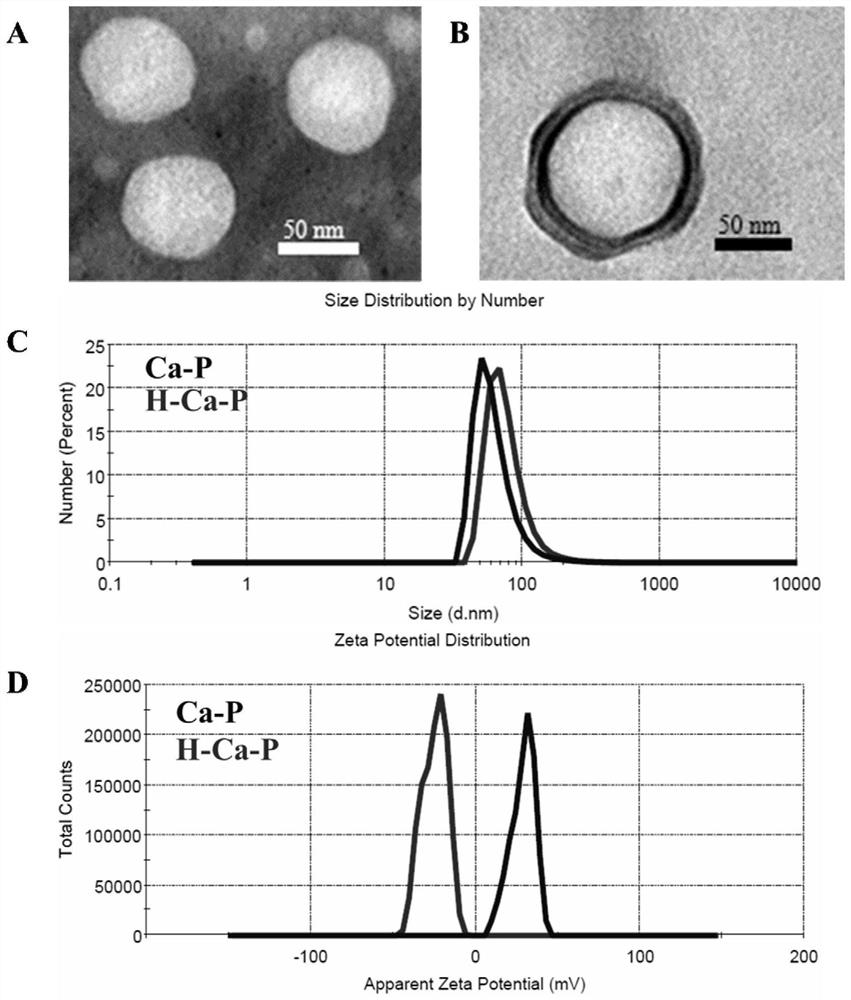
Calcium phosphate-lipid nano-drug co-delivery system consisting of low molecular weight heparin and prodrug of natural drug
ActiveCN110960507AInhibit EMT processInhibition formationHydroxy compound active ingredientsInorganic non-active ingredientsPhosphorylationNeo angiogenesis
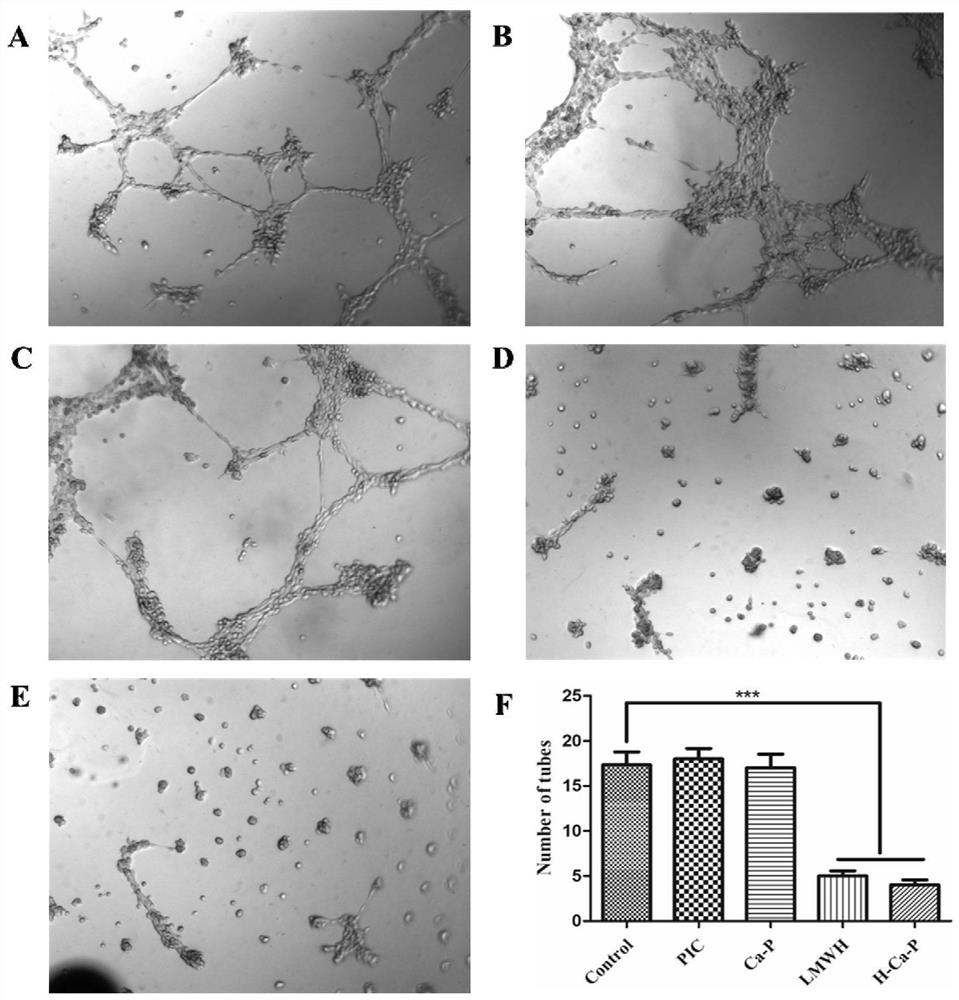
The invention relates to the field of pharmaceutical preparations, and relates to a calcium phosphate-lipid nano-drug co-delivery system consisting of low molecular weight heparin and a prodrug of a natural drug and a preparation method for the nano-drug co-delivery system. According to the nano-drug co-delivery system, nanoparticles prepared from a biodegradable lipid material are taken as carriers, and the phosphorylated prodrug PIC-POOH of the natural drug piceatannol (PIC) is physically entrapped, and the low molecular weight heparin (LMWH) adsorbs on the outer layers of the carriers by static electricity; and a nano preparation concentrates at a tumor site by utilizing the long circulating performance of the nano preparation and the enhanced permeability and retention (EPR) effect ofa solid tumor tissue, a related pathway for tumor cell metastasis is then regulated, angiogenesis is inhibited, and the anti-tumor metastasis action is exerted. Proved by assays, the nano-drug co-delivery system can inhibit the epithelial-mesenchymal transition (EMT) progress of tumor cells; proved by a tube formation assay, the nano-drug co-delivery system can significantly inhibit tumor angiogenesis; proved by in vivo administration evaluation, the nano-drug co-delivery system can reduce formation of lung metastasis on a mice model, and prolongs the survival time of tumor-bearing mice; and the nano-drug co-delivery system has an obvious anti-tumor metastasis effect, especially reduces triple negative breast cancer metastasis, and has high safety.
Owner:FUDAN UNIV
A low molecular weight heparin injection nursing operation scale plate
The invention relates to a scale disk for nursing operation of low-molecular weight heparin injection. The scale disk comprises a main disk body and meter moving double wings. The main disk body comprises a positioning disk, an inner circumferential rail, an outer circumferential rail, a scale sector plate, a pointer positioning rod, and a concentric positioning rod. The inner circumferential rail is arranged on the outer edge of the positioning disk. The scale sector plate is slidably configured between the inner circumferential rail and the outer circumferential rail. The meter moving double wings are slidably configured on the outer circumferential rail of the main disk body. Aimed at injection medicine characteristics of low-molecular weight heparin, horizontal, longitudinal, and circumferential distances of an injection position are accurately controlled around 20 mm. through the positioning disk, an injection range is positioned, a central positioning plate aligns at a basic injection point, an injection forbidden annular plate shields an injection forbidden region, the pointer positioning rod positions angles and longitudinal interval, and the concentric positioning rod positions distance and horizontal interval. After positioning holes of the two positioning rods are overlapped, a position most suitable for injection is accurately founded, so operation of a nurse is more accurate and rapid, and difficulty of self-operation of an intern doctor and a patient is effectively reduced.
Owner:青岛市中医医院
A kind of heparin lyase mutant and its recombinant expression method
ActiveCN111471669BHigh catalytic efficiencyImprove thermal stabilityAntibody mimetics/scaffoldsFermentationHeterologousBacteroides
The invention discloses a heparin lyase mutant and a recombinant expression method thereof, belonging to the technical field of bioengineering. The heparin lyase III provided by the invention has higher catalytic efficiency and thermal stability, and the catalytic efficiency of the mutant S264F / Y490K / D321N is 1.68 times higher than that of the original strain, which is more conducive to the preparation of low molecular weight heparin. The invention also performs heterologous recombination expression on the heparin lyase III from the Bacteroides proteus, and the intracellular enzyme activity can reach more than 4000U / L.
Owner:NANJING HANXIN PHARMA TECH CO LTD +1
Improved low-molecular weight heparin calcium raw material medicine and preparation thereof
ActiveCN106749769AHigh activityReasonable weight average molecular weightOrganic active ingredientsBlood disorderHigh dosesSodium nitrite
The invention belongs to the technical field of pharmaceutical preparations, particularly relates to a low-molecular weight heparin calcium raw material medicine and a preparation thereof, and aims at providing low-molecular weight heparin calcium and a low-molecular weight heparin calcium product to overcome the defects of the prior art. The raw material medicine is prepared by the processes of acid resin acidification, sodium nitrite pyrolysis, calcium oxide neutralization, anhydrous calcium chloride ethanol solution alcohol precipitation, and filtering and drying. Particularly, nitrous acid pyrolysis with relatively low pH area and relatively high dose is achieved in the presence of ethanol with a certain concentration range, the low-molecular weight heparin calcium preparation up to the standard is prepared, and a safe, effective, safe and quality-controlled low-molecular weight heparin calcium preparation is finally provided.
Owner:HAINAN UNIPUL PHARMA
Production and purification process of high-purity low-molecular-weight heparin sodium
The invention discloses a production and purification process of high-purity low-molecular-weight heparin sodium, and the proocess comprises the following steps of: S1, weighing 80-120g of crude heparin sodium, performing dissolving with purified water, controlling the temperature of the solution to be below 30DEG C, and conducting stirring for dissolving to prepare a 10%-20% (w / v) crude heparin sodium solution; S2, adding the crude heparin sodium solution in a NaCl solution with a concentration of 2% to prepare a 5%-10% (w / v) crude heparin sodium solution, adding alkaline protease, and carrying out heat preservation enzymolysis at 35-50DEG C for 3-6h to obtain an enzymatic hydrolysate; and S3, carrying out centrifugal separation on the enzymatic hydrolysate obtained in S2 to obtain a supernatant and a precipitate, and discarding insoluble impurities. According to the method, macromolecular substances such as residual protein in the produced low-molecular-weight heparin are interceptedby using an ultrafiltration method, so that the safety of the low-molecular-weight heparin is improved, and the low-molecular-weight heparin sodium is low in protein content, high in purity and highin yield.
Owner:浙江亚泰生物科技有限公司
Escherichia coli producing heparinase, construction method and application thereof
Belonging to the technical field of construction of genetic engineering bacteria, the invention provides a Escherichia coli BLR(DE3)-pBENT-H1 strain producing heparinase. The preservation number of the strain is CGMCC No.15819. The Escherichia coli producing heparinase provided by the invention can produce heparinase with enzyme activity of 2540U / L, the obtained heparinase is subjected to enzymolysis of heparin, the obtained ultra-low molecular weight heparin has a weight-average molecular weight of 1419Da, and the low molecular weight heparin has an Anti-FXa / Anti-FIIa titer ratio of 28.4.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Process for the preparation of low molecular weight heparin
The present invention provides an improved process for the preparation of Enoxaparin sodium. The process is simple, commercially viable and industrially advantageous.
Owner:BIOLOGICAL E LTD
Method for preparing nadroparin calcium and dalteparin sodium
ActiveCN112830980AChemical industryChemical/physical/physico-chemical microreactorsUltrafiltrationPhysical chemistry
The invention belongs to the field of preparation of low-molecular-weight heparin, and relates to a method for preparing nadroparin calcium and dalteparin sodium. Specifically, the invention relates to a method for preparing low-molecular-weight heparin, which comprises the steps of heparin sodium cracking, reduction, neutralization and ultrafiltration in a microchannel reactor, and the low-molecular-weight heparin is selected from nadroparin calcium and dalteparin sodium. According to the method, the selectivity of heparin sodium cracking reaction is improved, and the probability of repeated cracking is reduced, so that the increase of micromolecular heparin is avoided, the load of later ultrafiltration molecular weight screening is reduced, and the yield of the product is improved.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Calcium phosphate-lipid nano-drug co-delivery system composed of low molecular weight heparin and natural drug prodrug
ActiveCN110960507BInhibit EMT processInhibition formationHydroxy compound active ingredientsInorganic non-active ingredientsBreast cancer metastasisPhosphorylation
The field of pharmaceutical preparations of the present invention relates to a calcium phosphate-lipid nano-drug co-delivery system composed of low-molecular-weight heparin and natural drug prodrugs and a preparation method thereof. The present invention uses nanoparticles prepared from biodegradable lipid materials as a carrier , and physically encapsulates the phosphorylated prodrug PIC-POOH of the natural drug PIC, and electrostatically adsorbs LMWH on the outer layer, and utilizes the long-circulation properties of nano-preparations and the EPR effect of solid tumor tissues to enrich in tumor sites, thereby regulating tumor cell metastasis-related pathways , inhibit angiogenesis, anti-tumor metastasis. Experiments show that the drug co-delivery system can inhibit the EMT process of tumor cells, and can significantly inhibit tumor angiogenesis through tubule formation experiments. and prolong the survival period of tumor-bearing mice; it has obvious anti-tumor metastasis effect, especially reduces triple-negative breast cancer metastasis, and has good safety.
Owner:FUDAN UNIV
A low-molecular-weight heparin-modified bone-targeting liposome and its preparation method
ActiveCN111001011BGood curative effectImprove active targetingOrganic active ingredientsSkeletal disorderTumour metastasisBone targeting
Owner:CHINA PHARM UNIV
Tumor cell detection probe and preparation method and application thereof
InactiveCN110743020AProcess stabilityEasy to detectPowder deliveryNanoopticsTumor targetingSelf-assembly
The invention provides a tumor cell detection probe and a preparation method and application thereof. The tumor cell detection probe comprises a carrier, low molecular weight heparin modified cadmiumtelluride quantum dots loaded on the carrier and a tumor targeting ligand, wherein the carrier is a PEG-PLA block polymer. The PEG-PLA block polymer selected by the carrier in the tumor cell detectionprobe can be self-assembled to enable the tumor cell detection probe to form a nano structure, so that the tumor cell detection probe can stably exist in a body fluid environment, the tumor can be targeted based on the targeting effect of the tumor targeting ligand, the detection and diagnosis of the tumor can be realized through quantum dot imaging at the tumor part, the detection sensitivity ishigh, and the detection is accurate and efficient.
Owner:南京迪安医学检验所有限公司
Carboxylated derivatives of glycosaminoglycans and use as drugs
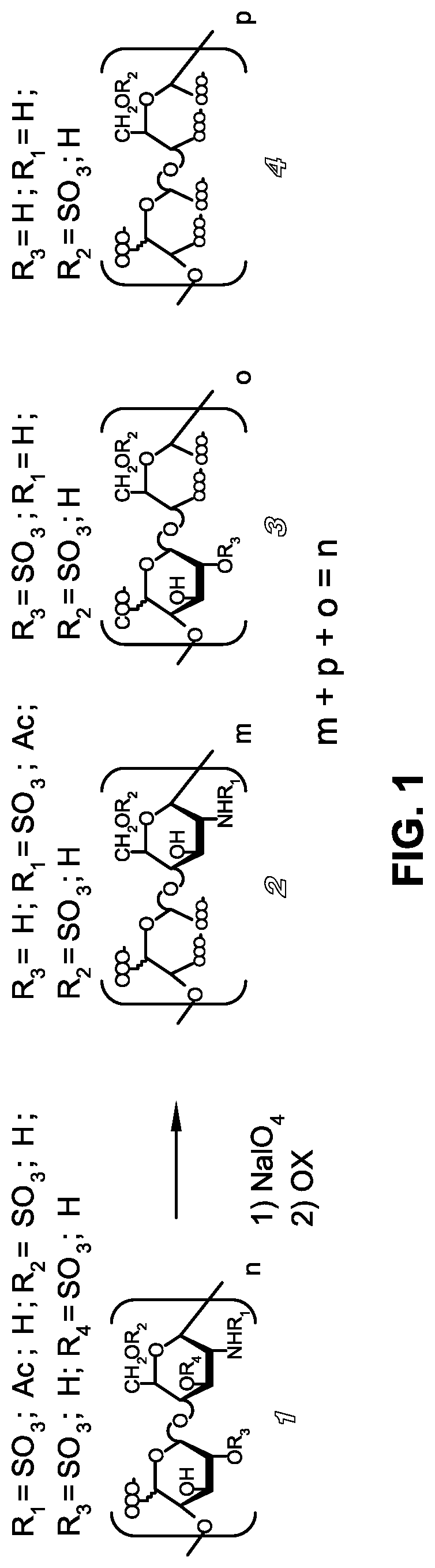
A glycosaminoglycan derivative endowed with heparanase inhibitory activity and antitumor activity, bearing carboxylate groups in positions 2 and 3 of at least part of the glycosaminoglycan residues, and to the process for preparing the same. The glycosaminoglycan derivatives of the present invention are generated starting from natural or synthetic glycosaminoglycans, preferably heparin or low molecular weight heparin, optionally 2-O- and 2-N-desulfated by two steps of oxidation. By the first oxidation, adjacent dials and optionally adjacent OH / NH2 of the glycosaminoglycan residues are converted to aldehydes and by the second oxidation said dialdehydes are converted to carboxylate groups. The first oxidation preferably leads to the cleavage of C2-C3 linkage of the ring of oxidable residues. The invention relates to a process for the preparation of said glycosaminoglycan derivatives and to their use as active ingredients of medicaments.
Owner:NOVAHEALTH BIOSYST LLC
Fusion expression of heparin lyase in bacillus subtilis and application of heparin lyase
ActiveCN113862248AStrong coagulation activityHigh anticoagulant activityBacteriaMicroorganism based processesHeterologousHeparin lyase
The invention discloses fusion expression of heparin lyase in bacillus subtilis and application of heparin lyase, and belongs to the technical field of bioengineering. According to the invention, bacillus subtilis is used as a host for heterologous expression of heparin lyase I from Bacteroides thetaiotaomicron, an oligopeptide sequence, an optimized promoter and a 5'UTR sequence are fused at an N terminal, a gene FhepIII of heparin lyase III is connected to a C terminal of a BhepI gene by using a GGGS oligopeptide, a fusion enzyme BhepI-FhepIII is constructed, and through comparison of heparin sodium splitting capacities of the BhepI and the FhepIII, it is found that the molecular weight of heparin obtained by the fusion enzyme can be as low as 1300Da or below, and the heparin lyase has stronger anticoagulant activity, and lays a foundation for industrial production of the heparin lyase in bacillus subtilis and preparation of low-molecular-weight heparin.
Owner:JIANGNAN UNIV
Camptothecin prodrug as well as preparation method and application thereof
PendingCN113952465AQuick releaseQuick release and killOrganic active ingredientsNanomedicineNanoparticleDrugs preparations
The invention discloses a camptothecin prodrug as well as a preparation method and application thereof, and belongs to the technical field of pharmaceutical preparations. According to the invention, a prodrug strategy is utilized, camptothecin is chemically bonded to a low molecular weight heparin (LMWH) molecular chain to form a prodrug, and the synthesized LMWH-CPT is self-assembled to form the nanoparticles by virtue of the hydrophobicity of the camptothecin and the hydrophilicity of the low molecular weight heparin. The camptothecin prodrug LMWH-CPT not only has the advantages of a polymer prodrug, but also the equilibrium constant pKa of camptothecin molecules in a nanoparticle core is remarkably increased by virtue of the unique hydrophobic interaction of the camptothecin molecules, so that the camptothecin prodrug LMWH-CPT is ensured to have a relatively high closed-loop rate after being stored in a neutral environment and being subjected to injection administration.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com