Fusion expression of heparin lyase in bacillus subtilis and application of heparin lyase
A technology of fusion expression and lyase, applied in the directions of lyase, carbon-oxygen lyase, application, etc., can solve the problems of low expression and limited application of heparin oligosaccharides, and achieve the effect of strong coagulation activity and high anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Inducible expression of BhepI in Bacillus subtilis
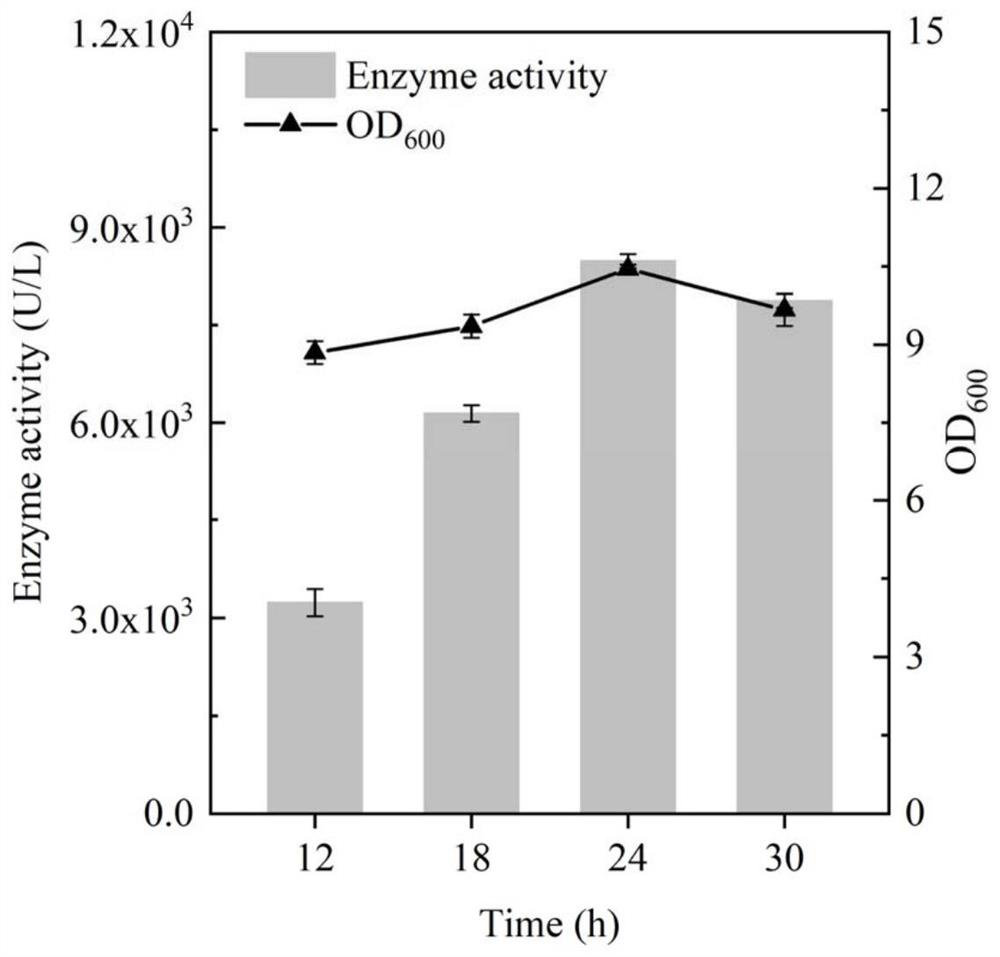
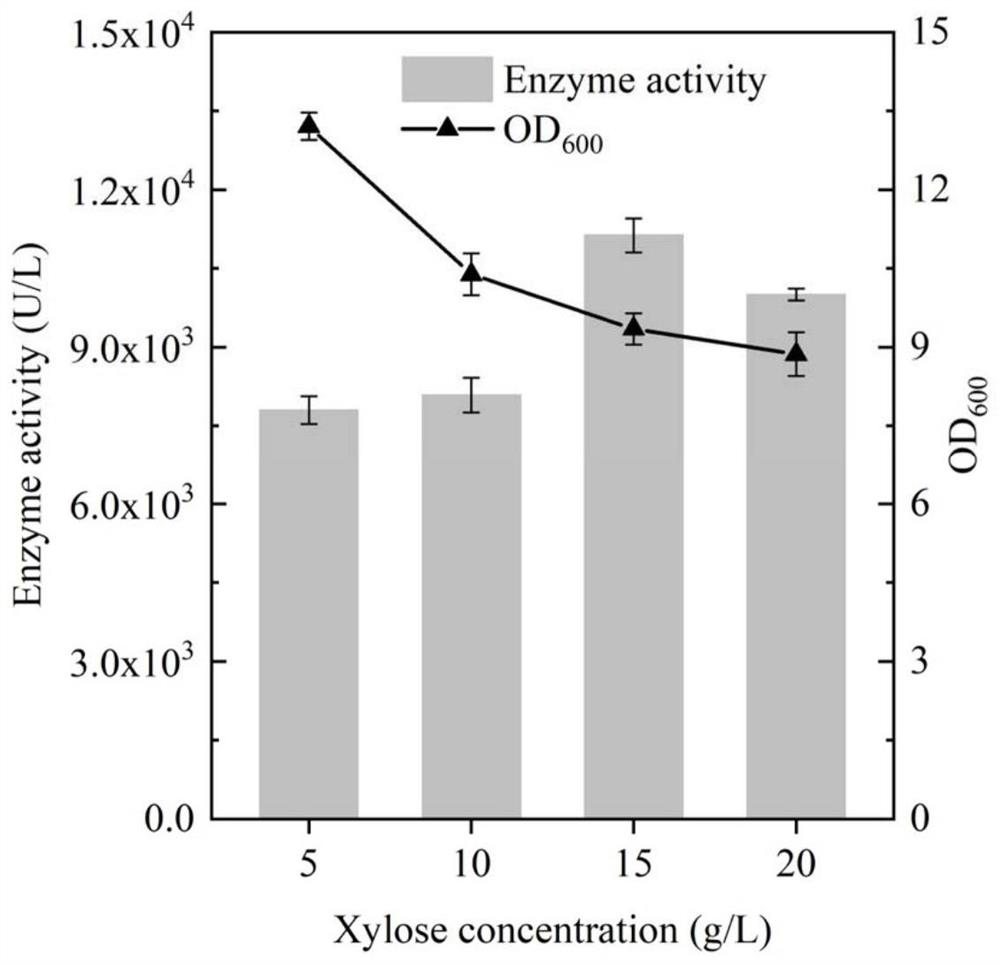
[0045] Standard PCR amplification system and procedures were used, and the primers used are listed in Table 1. Using the plasmid pET28a-BhepI as a template, primers STOP-BhepI-F and STOP-BhepI-R were designed to amplify and obtain a DNA fragment containing the gene BhepI encoding heparin lyase I. The resulting gene was inserted into the P of plasmid pSTOP1622 by Gibson assembly xylADownstream of the xylose-inducible promoter, the inducible recombinant plasmid pSTOP-BhepI was obtained, and transformed into B. subtilis WB600 cells to obtain the recombinant strain BSBH, and the culture time and the addition amount of the inducer were optimized for the recombinant bacteria. The resulting recombinant strain was inoculated into 3 mL of fresh LB liquid medium containing 20 μg / mL tetracycline, cultivated at 37 ° C and 220 rpm for 8-10 h to the mid-logarithmic phase, and then transferred to a 50 mL In a 250mL Erle...
Embodiment 2
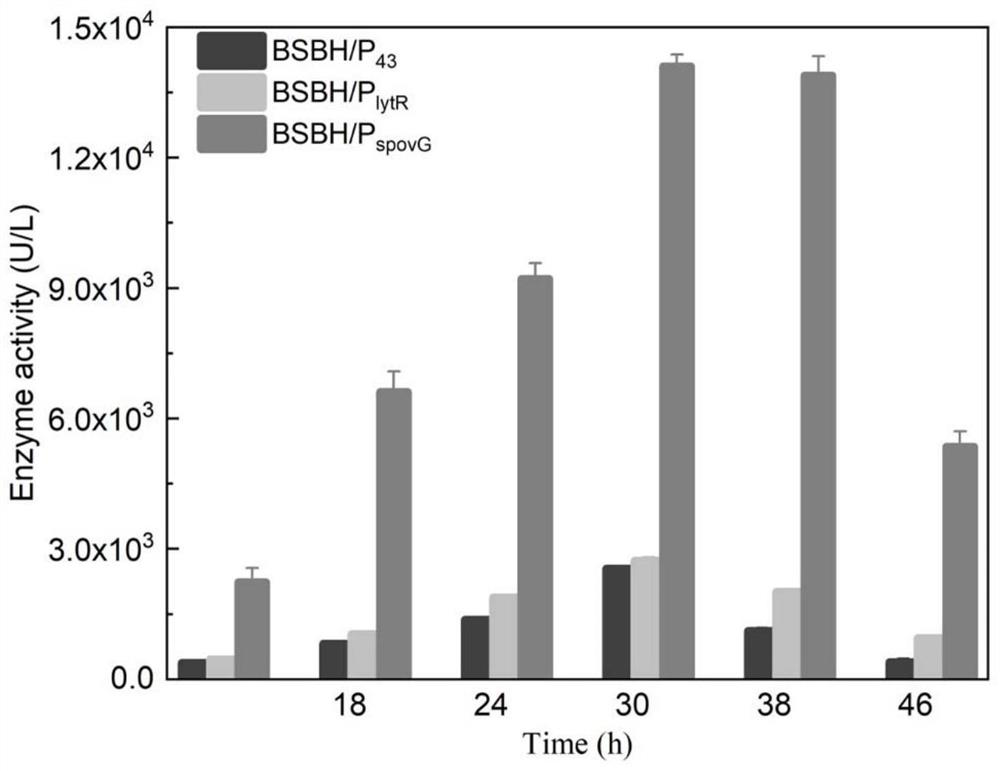
[0051] Example 2: Replacement of a constitutive promoter increases the constitutive expression level of BhepI
[0052] Selection of B. subtilis endogenous three promoters P spovG ,P lytR and P 43 , the P of the recombinant plasmid pSTOP-BhepI constructed in the above-mentioned Example 1 xylA The promoters were replaced by P spovG ,P lytR and P 43 Promoter. Design primer P 43 -F and P 43 -R, the primers used are shown in Table 1, and P 43 The gene sequence of the promoter, replacing the P of the pSTOP-BhepI plasmid by Gibson assembly xylA promoter, to obtain the recombinant plasmid P 43 -BhepI, obtain recombinant plasmid P in the same way spovG -BhepI and P lytR -Bhep I. And transferred to B. subtilis WB600 cells respectively to obtain recombinant strain BSBH / P spovG 、BSBH / P lytR and BSBH / P 43 . The resulting recombinant strain was inoculated into 3 mL of fresh LB liquid medium containing 20 μg / mL tetracycline, cultivated at 37 ° C and 220 rpm for 8-10 h to the...
Embodiment 3
[0054] Example 3: Optimizing the N-terminal sequence to improve the expression level of BhepI
[0055] On the basis of the recombinant plasmid constructed in Example 2 above, ten oligopeptide sequences were inserted into the N-terminus of the BhepI gene, and the N-terminal sequences are shown in Table 2. Using primers ybdD-F and ybdD-R to plasmid P spovG -BhepI is used as a template for full plasmid amplification to obtain recombinant plasmid P spovG -ybdD-BhepI, and so on, the primers used are shown in Table 1. Transform the constructed recombinant plasmids into B. subtilis WB600 cells to obtain 10 recombinant strains BSBH / P spovG -bltD, BSBH / P spovG -cspB, BSBH / P spovG -C4, BSBH / P spovG -yxjG, BSBH / P spovG -ydbD, BSBH / P spovG -valS, BSBH / P spovG -tufA, BSBH / P spovG -ybdD, BSBH / P spovG -yvyD and BSBH / P spovG -glnA. The resulting recombinant strain was inoculated into 3 mL of fresh LB liquid medium containing 20 μg / mL tetracycline, cultivated at 37 ° C and 220 rpm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com