Preparation method of oligosaccharide containing N-acetylated structure heparin
A technology of heparin oligosaccharide and acetylation, which can be used in measurement devices, instruments, scientific instruments, etc., and can solve problems such as difficult preparation and structure determination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]1) Dissolve 200 mg low molecular weight heparin in 2.0ml, 0.1 M, pH 7.0 sodium acetate buffer solution (containing 0.1mM calcium acetate and 100μg / ml BSA), add 50 mIU heparinase Ⅰ, and place in a 37°C water bath After 12 hours of enzymatic hydrolysis, add 50mIU heparanase I to continue enzymatic hydrolysis for 12 hours; then heat to 100°C and boil for 3 minutes to terminate the reaction, centrifuge at 10,000 rpm for 10 minutes, take the supernatant, and freeze-dry;
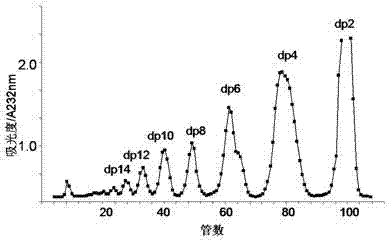
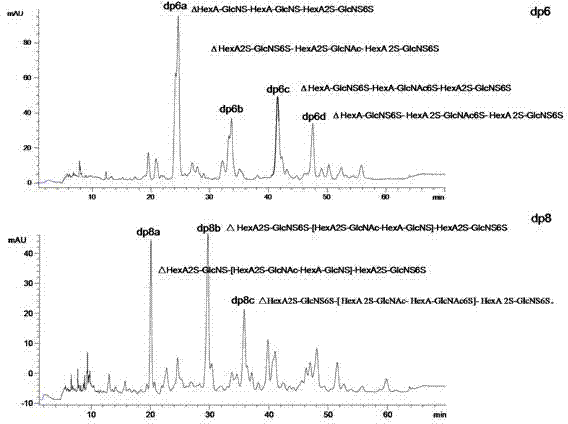
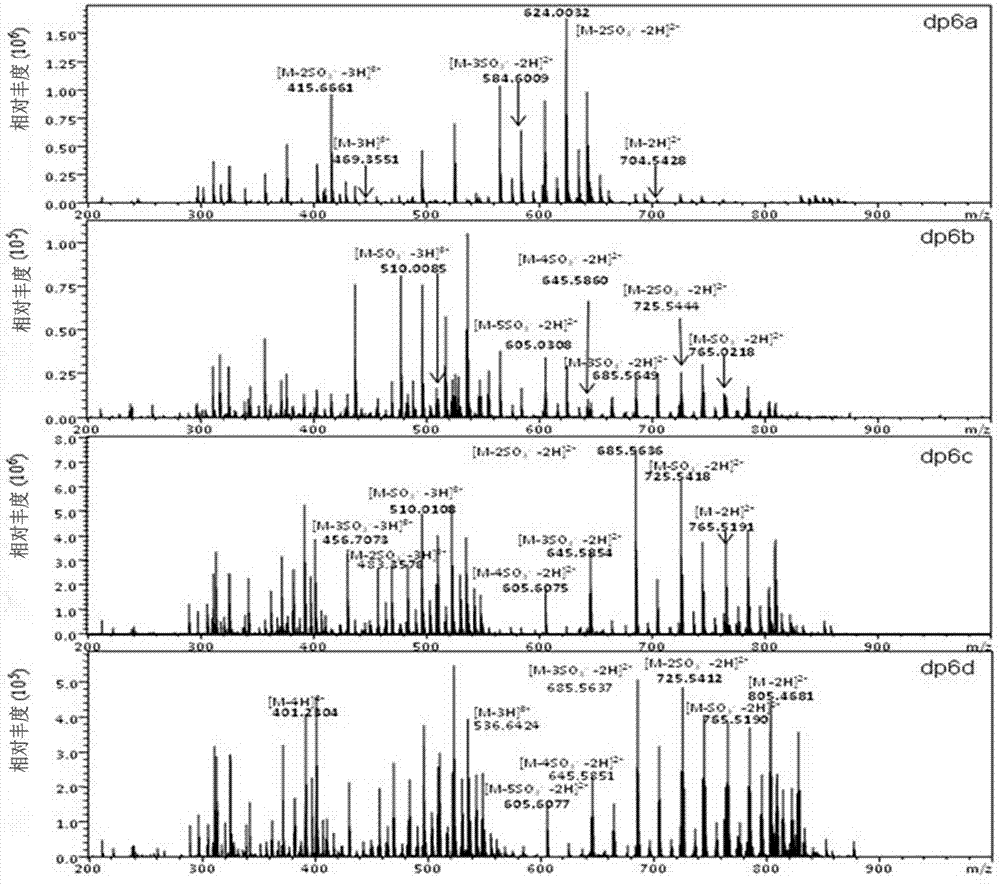
[0034] 2) Take the freeze-dried sample and dissolve it with 1ml 0.2M ammonium bicarbonate, load the sample to a series Bio-Gel chromatography column, elute with 0.2M ammonium bicarbonate, and collect it with an automatic collector at a flow rate of 0.2ml / min; the separated oligosaccharides Detect with a UV spectrophotometer at a detection wavelength of 232 nm; sequentially collect the sugar fragments corresponding to each chromatographic peak, heat at 55°C for 24h to volatilize ammonium bicarbonate, and freez...
Embodiment 2
[0048] 1) Dissolve 100 mg low molecular weight heparin in 1.0ml, 0.1 M, pH 7.0 sodium acetate buffer solution (containing 0.1mM calcium acetate and 100μg / ml BSA), add 50 mIU heparanase Ⅰ, and place in a 37°C water bath After 12 hours of enzymatic hydrolysis, add 50mIU heparanase I to continue enzymatic hydrolysis for 12 hours; then heat to 100°C and boil for 3 minutes to terminate the reaction, centrifuge at 10,000 rpm for 10 minutes, take the supernatant, and freeze-dry;
[0049] 2) Take the freeze-dried sample and dissolve it with 1ml 0.2M ammonium bicarbonate, load the sample to a series Bio-Gel chromatography column, elute with 0.2M ammonium bicarbonate, and collect it with an automatic collector at a flow rate of 0.2ml / min; the separated oligosaccharides Detect with a UV spectrophotometer at a detection wavelength of 232 nm; sequentially collect the sugar fragments corresponding to each chromatographic peak, heat at 55°C for 24h to volatilize ammonium bicarbonate, and free...
Embodiment 3
[0063] 1) Dissolve 300 mg low molecular weight heparin in 1.0ml, 0.1 M, pH 7.0 sodium acetate buffer solution (containing 0.1mM calcium acetate and 100μg / ml BSA), add 50 mIU heparanase Ⅰ, and place in a 37°C water bath After 12 hours of enzymatic hydrolysis, add 50mIU heparanase I to continue enzymatic hydrolysis for 12 hours; then heat to 100°C and boil for 3 minutes to terminate the reaction, centrifuge at 10,000 rpm for 10 minutes, take the supernatant, and freeze-dry;
[0064] 2) Take the freeze-dried sample and dissolve it with 1ml 0.2M ammonium bicarbonate, load the sample to a series Bio-Gel chromatography column, elute with 0.2M ammonium bicarbonate, and collect it with an automatic collector at a flow rate of 0.2ml / min; the separated oligosaccharides Detect with a UV spectrophotometer at a detection wavelength of 232 nm; sequentially collect the sugar fragments corresponding to each chromatographic peak, heat at 55°C for 24h to volatilize ammonium bicarbonate, and freeze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com