Heparin lyase mutant and recombinant expression method thereof
A technology for lysing enzymes and mutants, applied in the field of bioengineering, can solve the problems of limiting the application of heparin oligosaccharides, low thermal stability, and low catalytic efficiency, and achieve high catalytic efficiency and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The construction of embodiment 1 recombinant escherichia coli
[0036] Using the Bacteroidesthetaiotaomicron genome as a template, primers were designed, and a standard PCR amplification system and program was used to amplify and obtain the gene encoding heparin lyase III shown in SEQ ID NO.1. The resulting gene was connected to the pET-28a plasmid XhoI and NdeI restriction sites to obtain a recombinant plasmid, which was introduced into Escherichia coli BL21 (DE3) to obtain a recombinant Escherichia coli strain.
Embodiment 2
[0037] The shaking flask culture of embodiment 2 recombinant escherichia coli
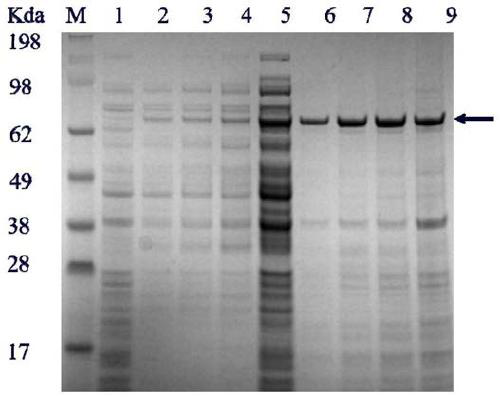
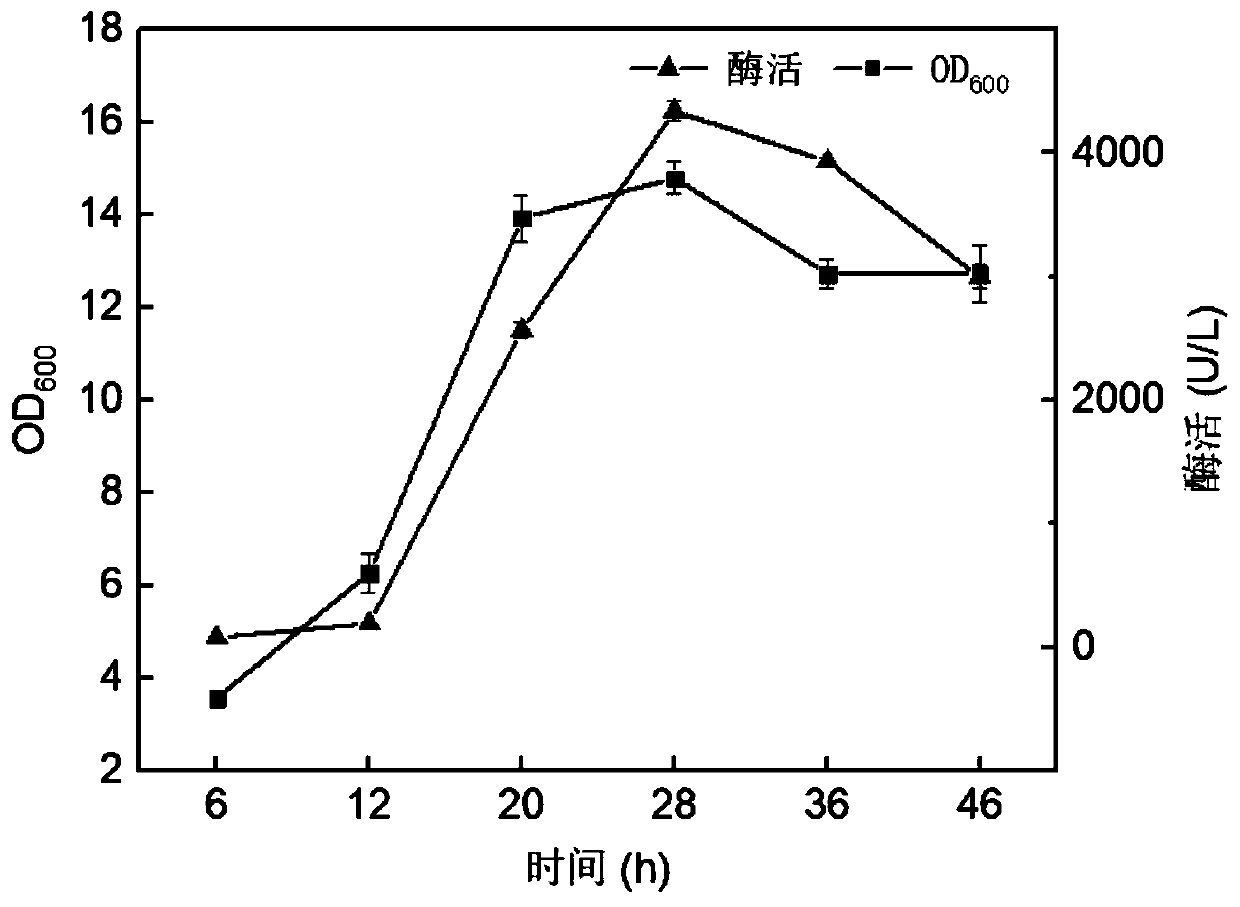
[0038] The recombinant Escherichia coli constructed in the above-mentioned Example 1 or the control bacteria were inoculated in LB medium, and the control bacteria were obtained by directly introducing the pET-28a empty plasmid into Escherichia coli BL21 (DE3). After overnight culture at 37°C, the recombinant Escherichia coli and control bacteria were respectively transferred to 250mL Erlenmeyer shake flasks filled with LB medium, cultured at 30°C for 1 hour, and induced by adding IPTG with a final concentration of 0.5mM, and cultured at 27°C 48h. Centrifuge the fermentation broth at 6800rpm for 10min at 4°C, discard the supernatant, resuspend the bacteria 2-3 times with 20mM Tris-HCl, break it with an ultrasonic cell disruptor, and centrifuge at 12000rpm for 20min at high speed to obtain the crude enzyme in the cell. The crude enzyme was eluted with 100mM imidazole to obtain pure enzyme after pas...
Embodiment 3
[0043] Embodiment 3 thermostability improves the acquisition of mutant
[0044]In order to improve the thermal stability of heparin lyase III, saturation mutations were carried out at the S264, D321, and Y490 sites. Using the recombinant plasmid carrying the gene encoding the unmutated heparin lyase III constructed in Example 1 as a template, a standard PCR program was used to amplify, and the primers used are shown in Table 1. Introduce the PCR amplification product into the competent cells of the large intestine, place it on ice for 30 minutes, heat shock at 42°C for 90 seconds, add LB medium and incubate at 37°C for 50 minutes, spread it on the Karna plate, and let it stand at 37°C Cultivate for 10-12 hours, select a suitable single colony and sequence to verify that the correct transformants are obtained. The cultivation method of the transformant and the method of obtaining the mutant pure enzyme are shown in Example 2. The obtained mutant pure enzyme was placed in 50mM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com