Heparin disaccharide mixture and preparation method and application thereof
A mixture and heparin technology, applied in biochemical equipment and methods, microbiological determination/inspection, measuring devices, etc., can solve problems affecting heparin enzyme chromatographic analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the preparation of standard disaccharide mixture
[0070] Step 1: Enzyme I degradation of heparin: Take 200mg heparin, dissolve in 2ml Tris-HCl (50mM, containing CaCl210mM, pH7.0) buffer solution, add heparinase I (50mM Tris-HCl solution, containing CaCl210mM, pH7.0) 0) 12IU, enzymatic hydrolysis at room temperature for 24 hours;
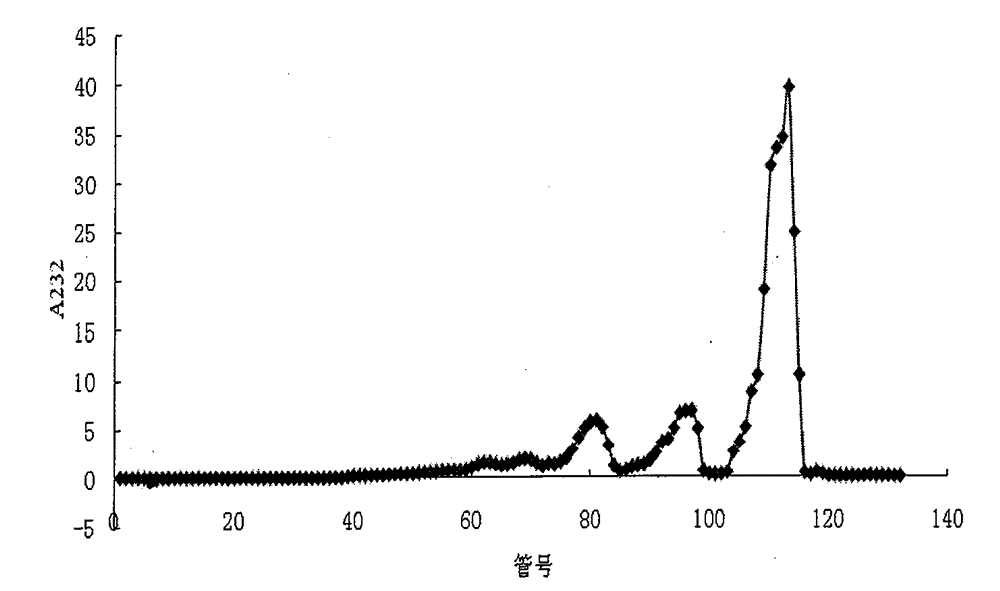
[0071] Step 2: Separation of two and four sugar fragments: take the degradation product of enzyme I, put it on a Bio-Gel P6 column (1.5×100cm), equilibrate and elute with 0.2M ammonium bicarbonate, flow rate 0.3ml / min, divide Collect, collect 130 tubes altogether, every tube 1.5ml, measure A232 value (A232 refers to the absorption value of 232nm that uses 1cm optical path cuvette to measure on the spectrophotometer), plots the number of elution tubes with A232 value ( Taking the number of elution tubes as the X-axis and A232 as the Y-axis to make a graph), the elution profile shows three main peaks, see figure 1 , from back to...
Embodiment 2
[0074] The labeled disaccharide mixture is disaccharide mixture 1 or disaccharide mixture 2 or a mixture of both. Embodiment 2: Identification and content determination of disaccharide mixture composition:
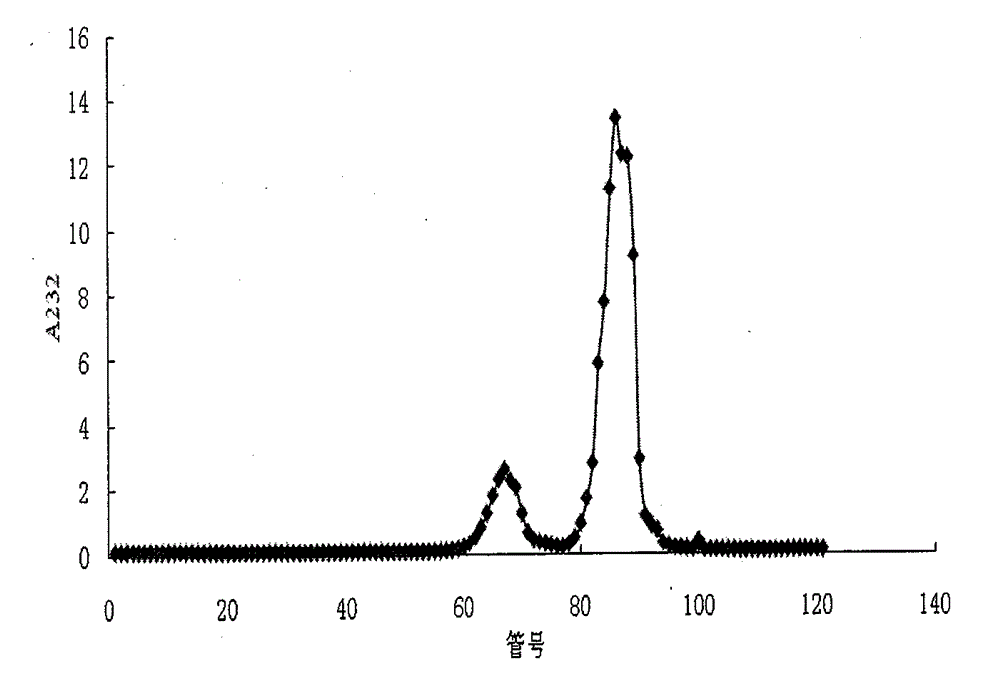
[0075] The SAX-HPLC method is adopted, and the chromatographic conditions are shown in Table 1. The elution gradient and the peak time of disaccharides are shown in Tables 2 and 3, respectively. The SAX-HPLC spectra of the two disaccharide mixtures are shown in Table 1. Figure 4 , 5 , the molar percentage of disaccharides is indicated on the graph. In addition to the absorption peaks of the 8 kinds of heparin disaccharides in the chromatogram, there are several other tiny absorption peaks, which leads to the sum of the proportions of the 8 kinds of disaccharides in the mixture being less than 100% after the peak area integration and quantification. These small absorption peaks may be trace amounts of disaccharides, trisaccharides, and tetrasaccharides that are difficult...
Embodiment 3
[0086] Example 3: Detection of the presence of heparin disaccharide-acting enzymes using a mixture of disaccharides
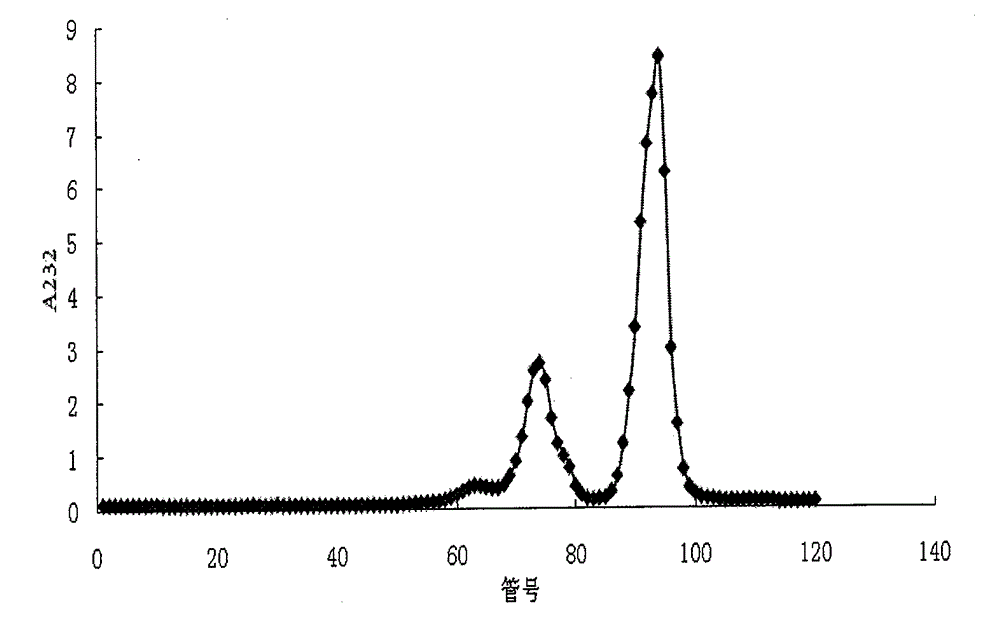
[0087] Disaccharide mixture 1, in Tris-HCl solution (50mM, containing CaCl 210mM, pH7.0) to prepare a 1mg / ml solution, add heparanase samples 1, 2, and 3 that may contain heparin disaccharide-acting enzyme impurities, react at room temperature for 24 hours, measure the SAX-HPLC disaccharide spectrum, observe Changes in the chromatographic peak area of each component. As a result, it was found that the chromatographic peak areas of disaccharide components in sample 1 did not change, which indicated that the enzyme did not contain heparin disaccharide-acting enzyme; in sample 2, the disaccharide I-S chromatographic peak area decreased, while the disaccharide II-S chromatographic peak area increased ,See Figure 6 , which means that it contains an enzyme that can catalyze the removal of the 2-sulfate group in α-ΔUA-2S-[1→4]-GlcNS-6S from the I-S disaccharide; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com