Pyraclostrobin and method for economically synthesizing same
An economical technology of pyraclostrobin, applied in the field of economical synthesis of pyraclostrobin, can solve the problems of high reaction temperature, low yield, uneconomical, etc., achieve high purity, high material utilization rate, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
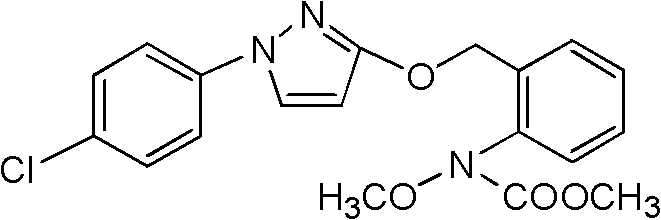
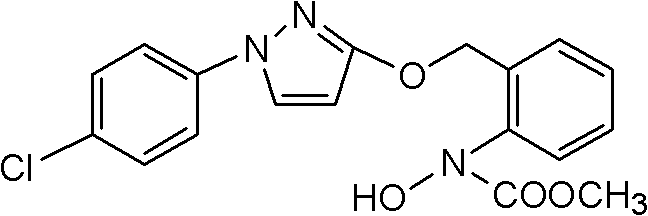
[0017] Add 78.8g (0.2mol) N-hydroxyl-N-2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]phenylcarbamate, 450mL acetone into a 1L four-necked flask, 41.9g (0.3mol) of anhydrous sodium carbonate, stirred at room temperature (10-30°C) for 0.5 hours, added dropwise 30.6g (0.24mol) of dimethyl sulfate at 25°C, after the dropwise addition, at a temperature of 25°C React for 2 hours, wash with water to neutralize, remove the solvent, add isopropanol to immerse, cool at 0-5 ° C, stand for 1 hour to recrystallize, and suction filter to obtain 77.7g of white crystals, the content of which is determined by HPLC (external standard) is 98.1%. Yield 98.3%, mp: 65°C, 1H NMR (300MHz, CDCl 3 ): δ: 3.8 (s, 3H, OCH 3 ), 5.9 (d, 1H, -PyH), 7.2-7.7 (m, 9H, ArH).
Embodiment 2
[0019] Add 78.8g (0.2mol) N-hydroxyl-N-2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]phenyl carbamate in 1L four-necked bottle, 1,2 - 450 mL of dichloroethane, 41.9 g (0.3 mol) of anhydrous sodium carbonate, stirred at room temperature for 0.5 hours, and added dropwise 30.6 g (0.24 mol) of dimethyl sulfate at 25°C. React for 2 hours, wash and neutralize with water, remove the solvent, add methanol or ethanol to immerse, cool at 0-5°C, let stand for 1 hour to recrystallize, and suction filter to obtain 77.2g of white crystals, the content of which was determined by HPLC (external standard) was 97.6%. The rate is 97.1%.
Embodiment 3
[0021] Add 78.8g (0.2mol) N-hydroxyl-N-2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]phenylcarbamate to a 1L four-neck flask, 450mL butanone , 30.7g (0.22mol) of anhydrous potassium carbonate, stirred at room temperature for 0.5 hours, added dropwise 28g (0.22mol) of dimethyl sulfate at 25°C, after the dropwise addition, reacted at a temperature of 25°C for 2 hours, washed with water and neutralized , remove the solvent, add n-butanol to immerse, cool at 0-5 ° C, stand for 1 hour to recrystallize, and suction filter to obtain 77.0 g of white crystals, the content of HPLC (external standard) is 97.2%, and the yield is 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com