Camptothecin prodrug as well as preparation method and application thereof
A camptothecin and prodrug technology, applied in the field of camptothecin-low molecular weight heparin prodrug self-assembly nano-delivery system, can solve problems such as poor water solubility, unstable active ester structure, and low loop closure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Camptothecin Prodrug LMWH-CP
[0027] 1. Synthesis of intermediate product LMWH-ADH
[0028] Accurately weigh 30 mL (4 mg / mL) of LMWH (MW=4500 Da, Nanjing Nanda Pharmaceutical Co., Ltd.) and add it to an appropriate amount of phosphate buffer solution with pH 6.8. After it is fully dissolved, add ADH 141.10 mg, EDCI 155.28 mg, HOBT109.59 mg, stirred at room temperature, adjusted the reaction system with 0.1M hydrochloric acid to make the pH value about 6.7, and reacted for at least 24 hours. The crude product obtained was dialyzed against distilled water in order to remove remaining reactants as well as small molecule by-products. The dialyzed filtrate was filtered and lyophilized to obtain the intermediate product LMWH-ADH.
[0029] 2. Synthesis of intermediate product CPT-OA
[0030] Accurately weigh camptothecin 100mg (0.3mmol), DMAP 73.30mg (0.6mmol), EDCI 93.17mg (0.6mmol), OA 104.52mg (0.6mmol) in a dry round bottom flask, add anhydrous dichloromet...
Embodiment 2
[0034] Preparation of LMWH-CPT nanoparticles
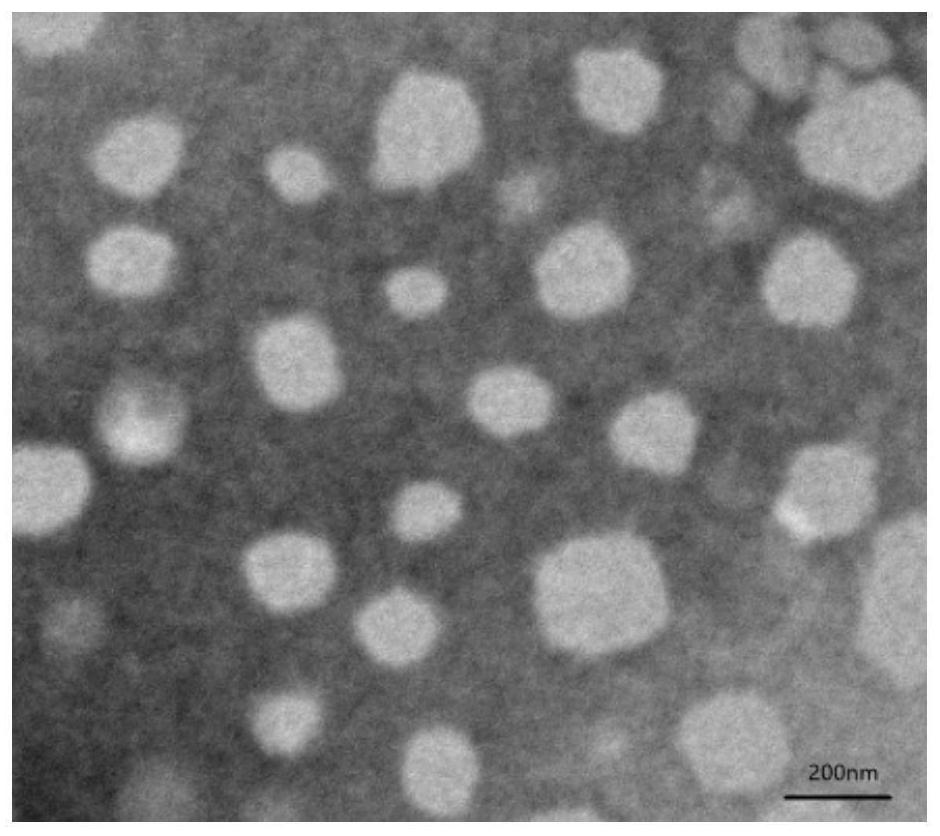
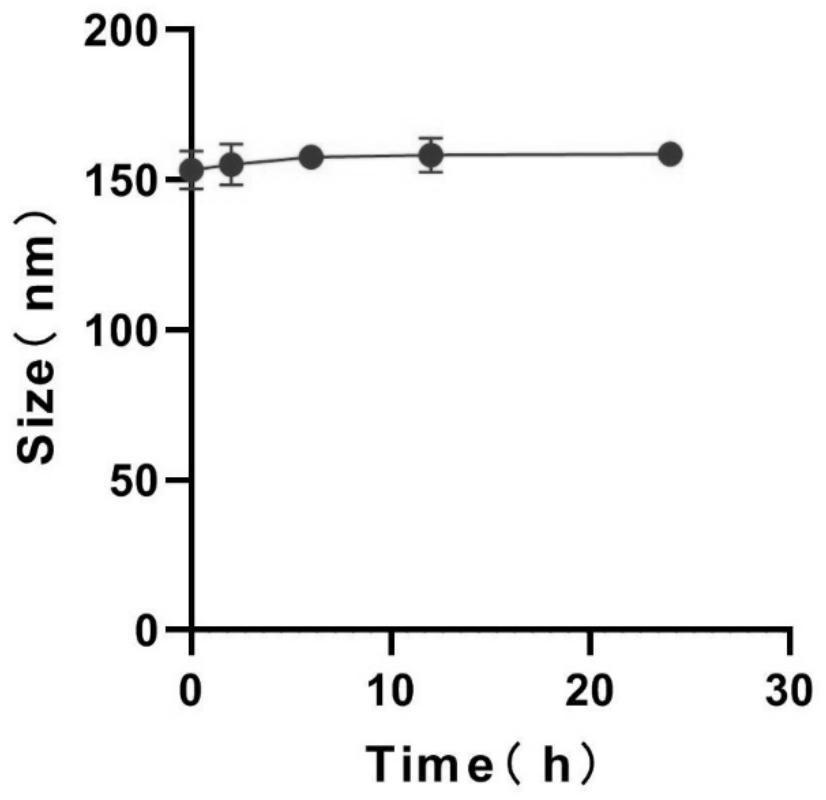
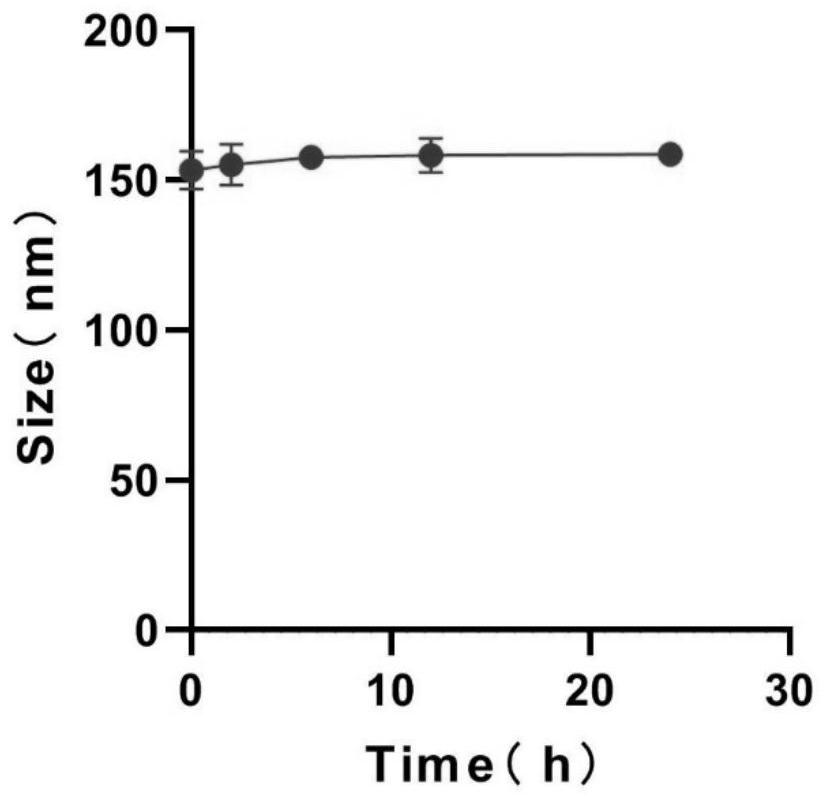
[0035] Precisely weigh 10 mg of camptothecin prodrug LMWH-CPT, dissolve it in 2 mL of tetrahydrofuran solution, drop it into 10 mL of ultrapure water while stirring with a magnetic stirrer, and stir until the tetrahydrofuran is completely volatilized, The probe was sonicated for 30 minutes in an ice bath (power 200W, working 1s, intermittent 2s), and then passed it through a 0.45 μm filter membrane to obtain a light yellow LMWH-CPT nanoparticle solution with Tyndall effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com