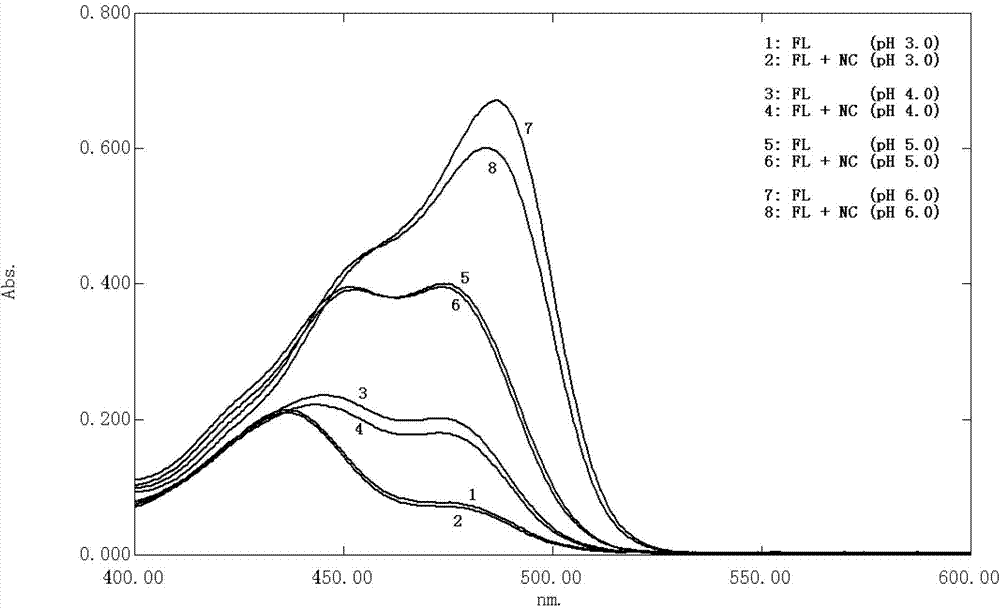
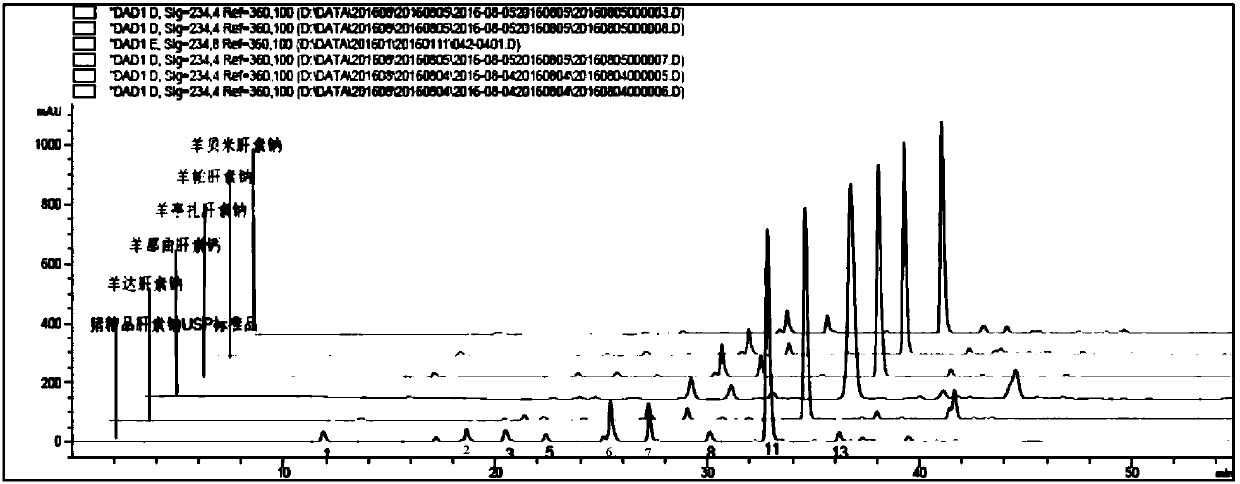
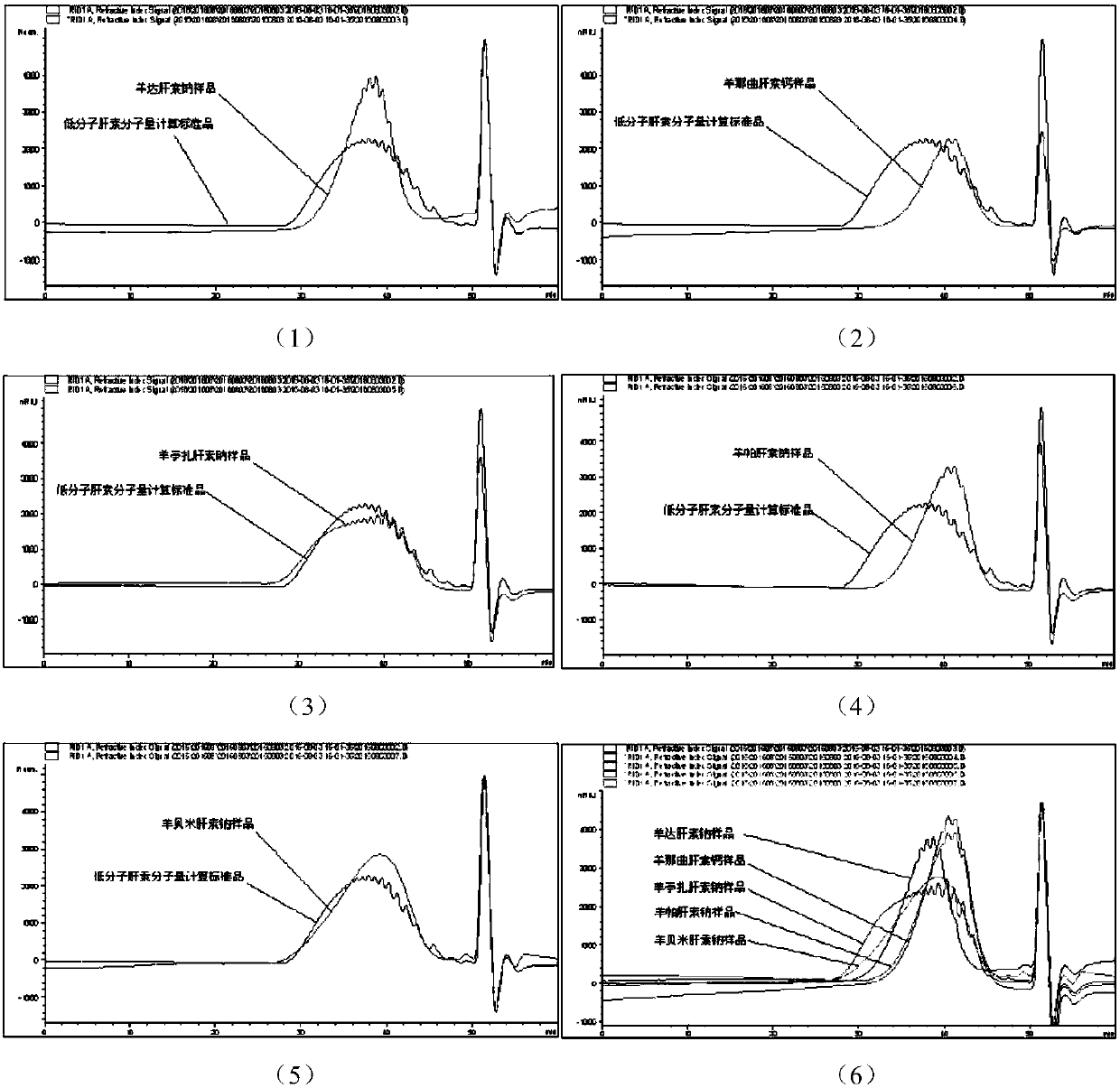
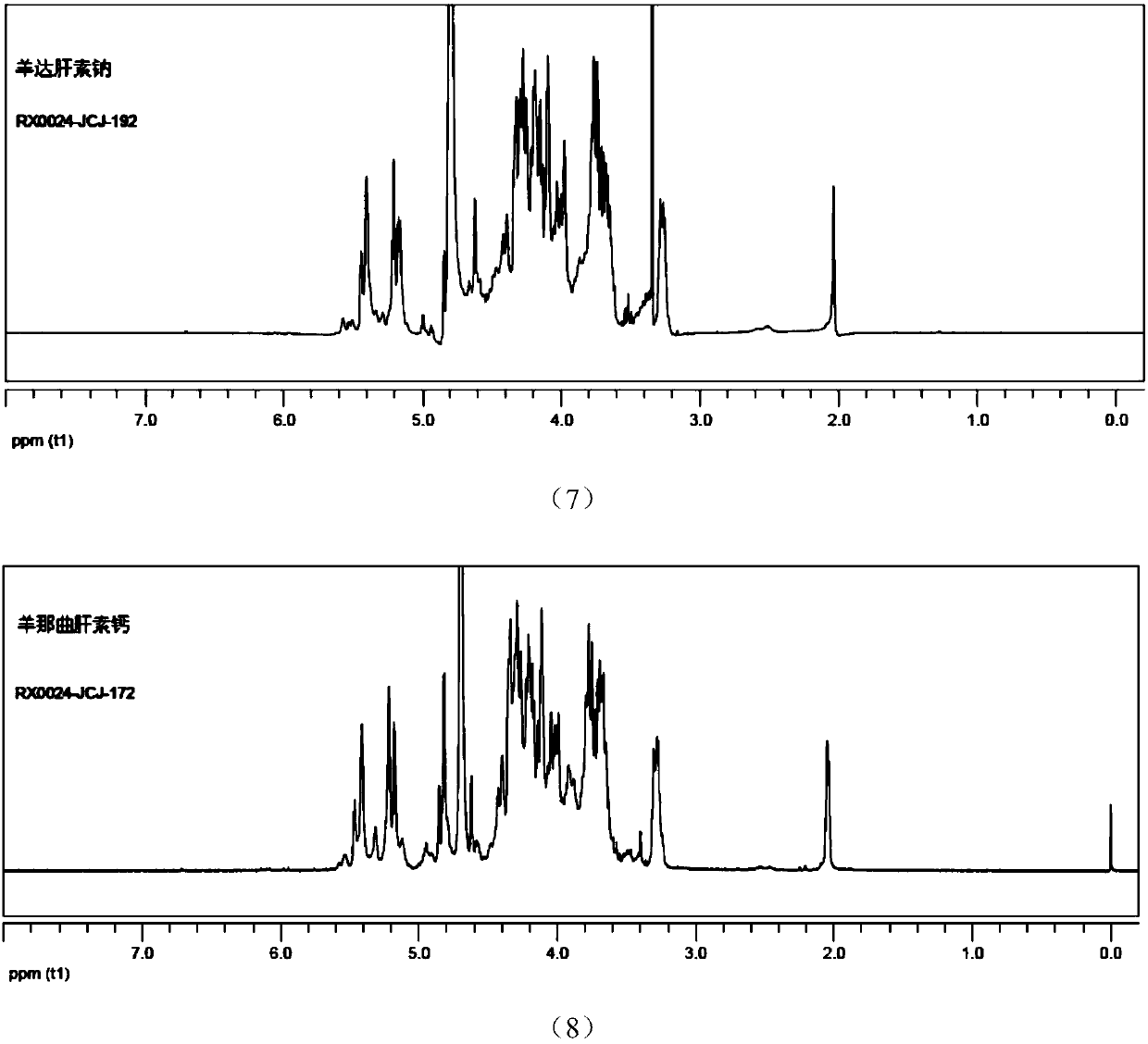
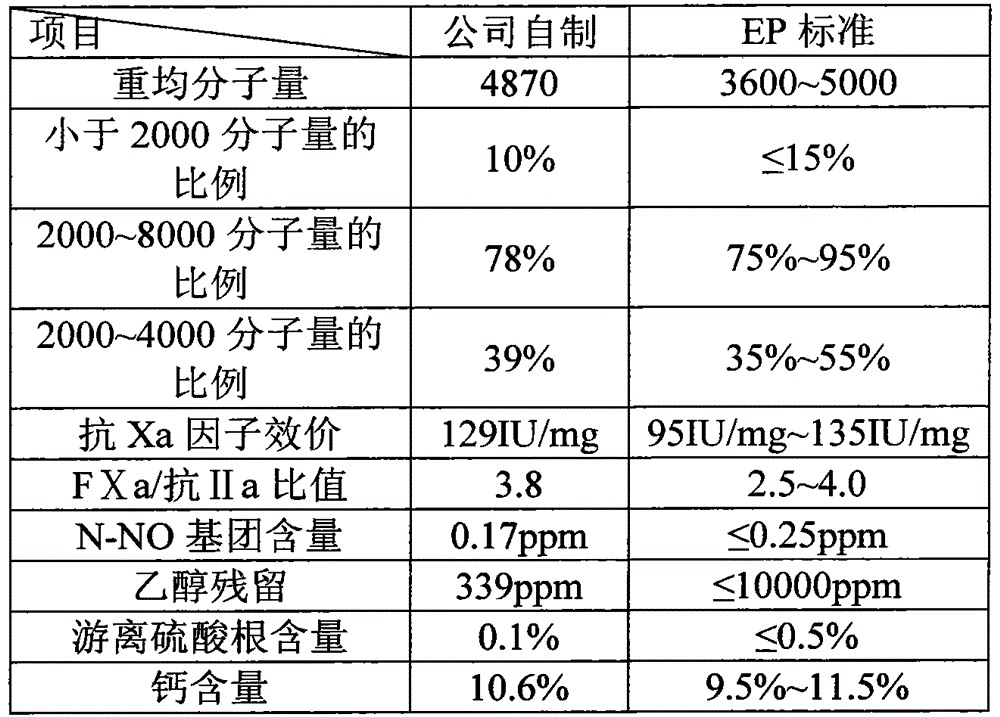
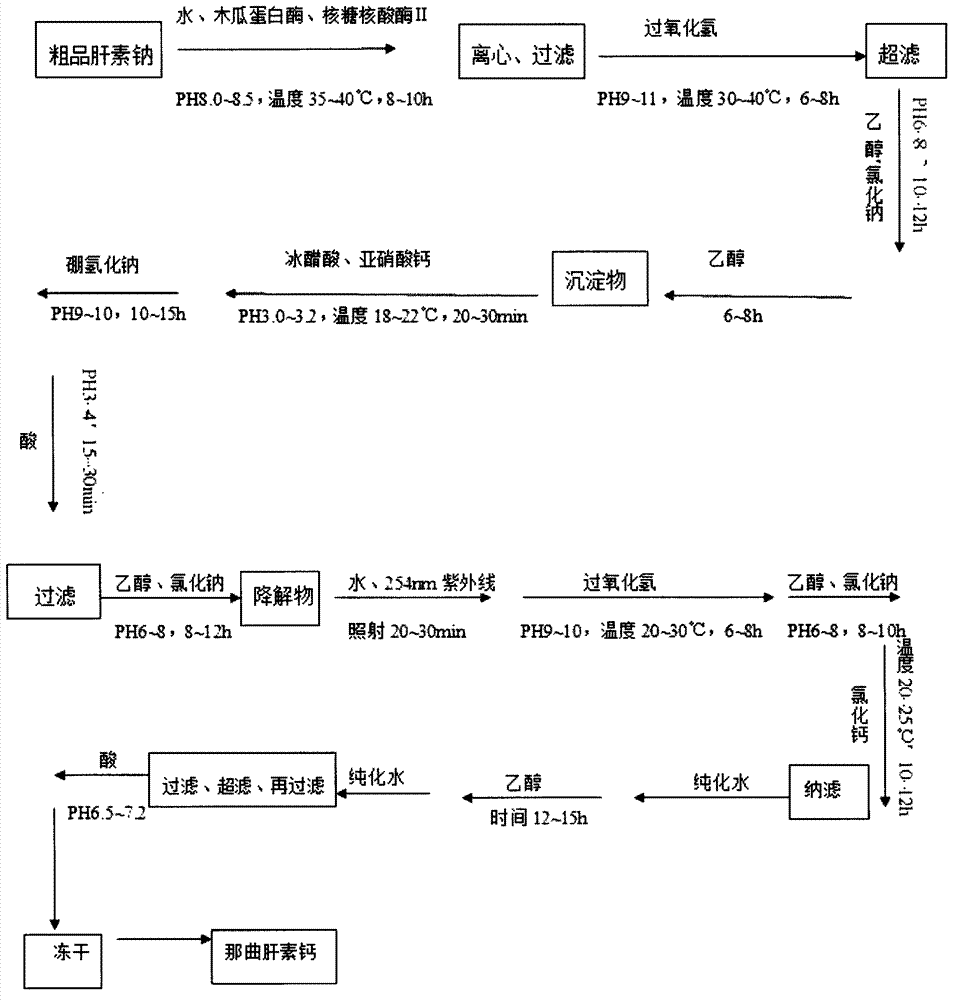
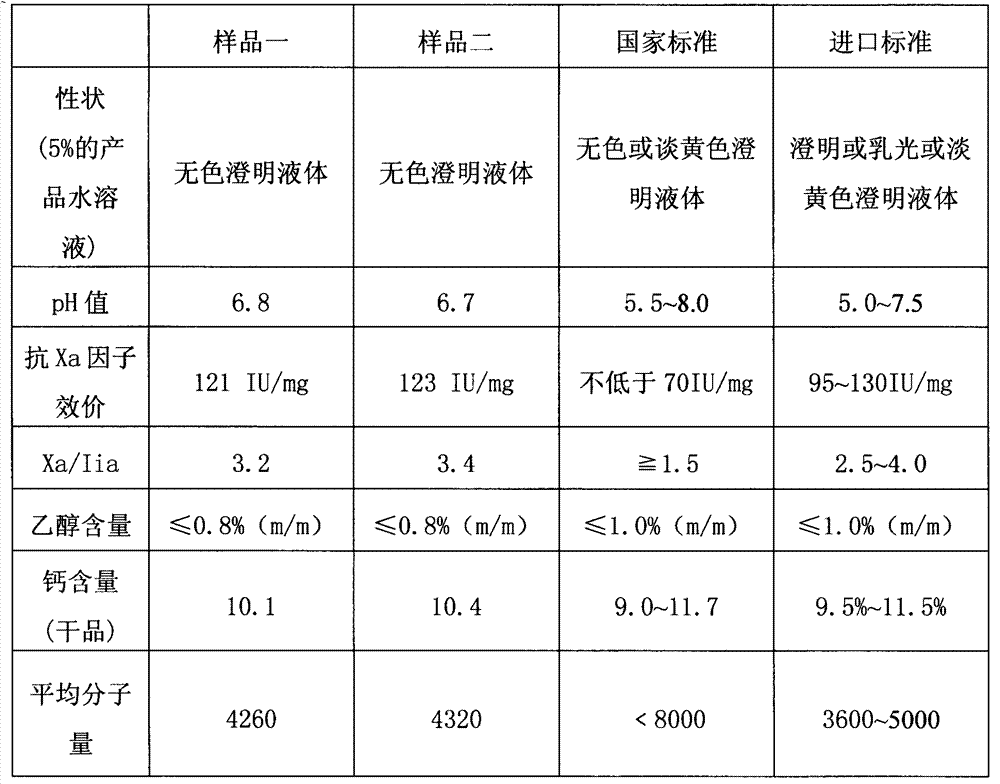
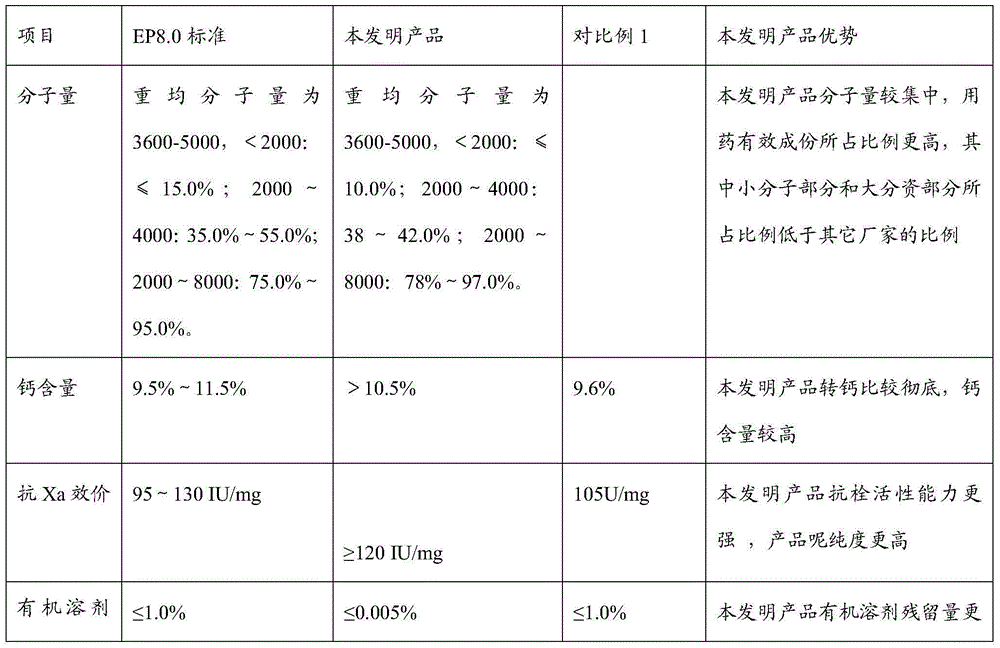
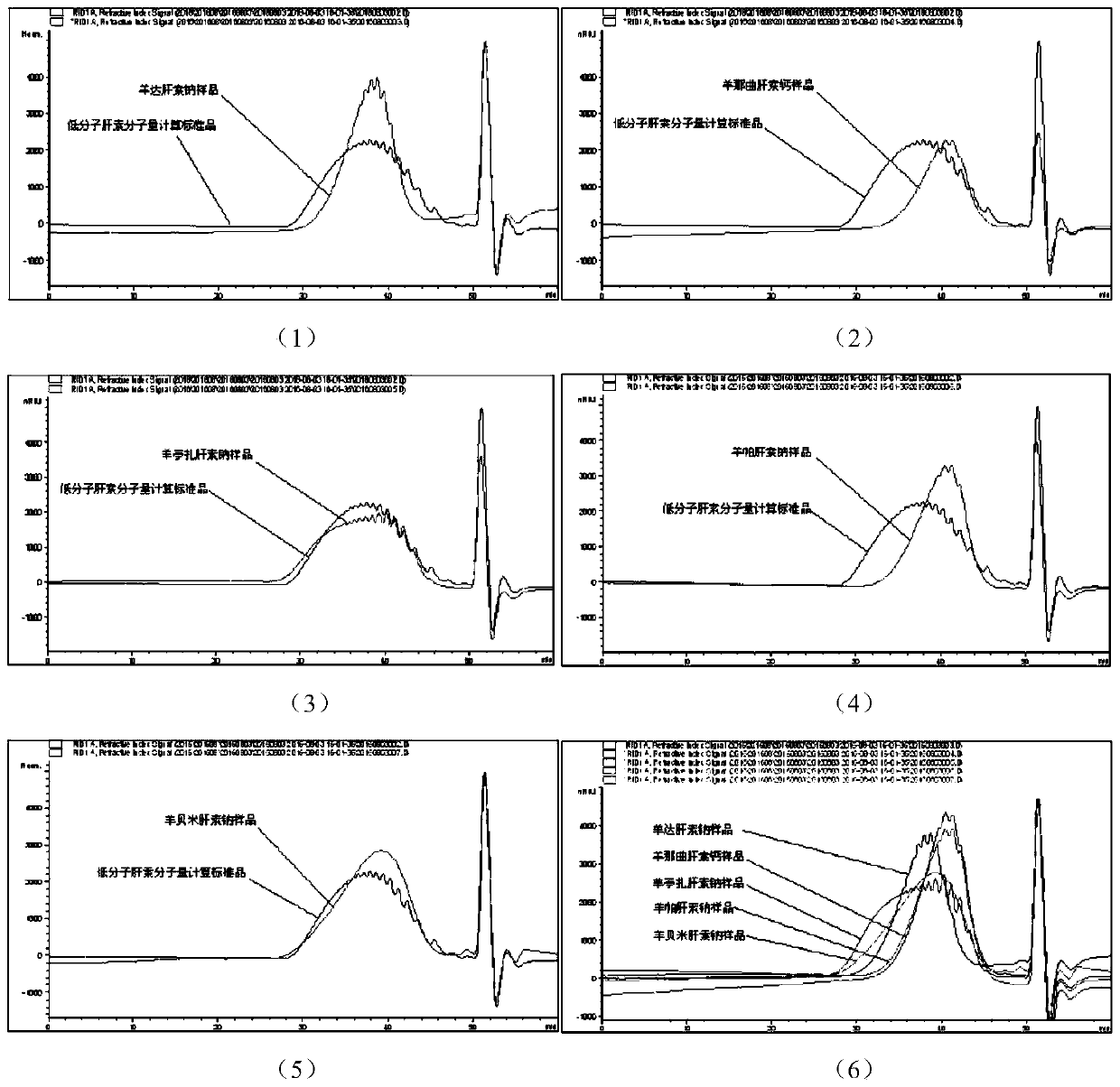
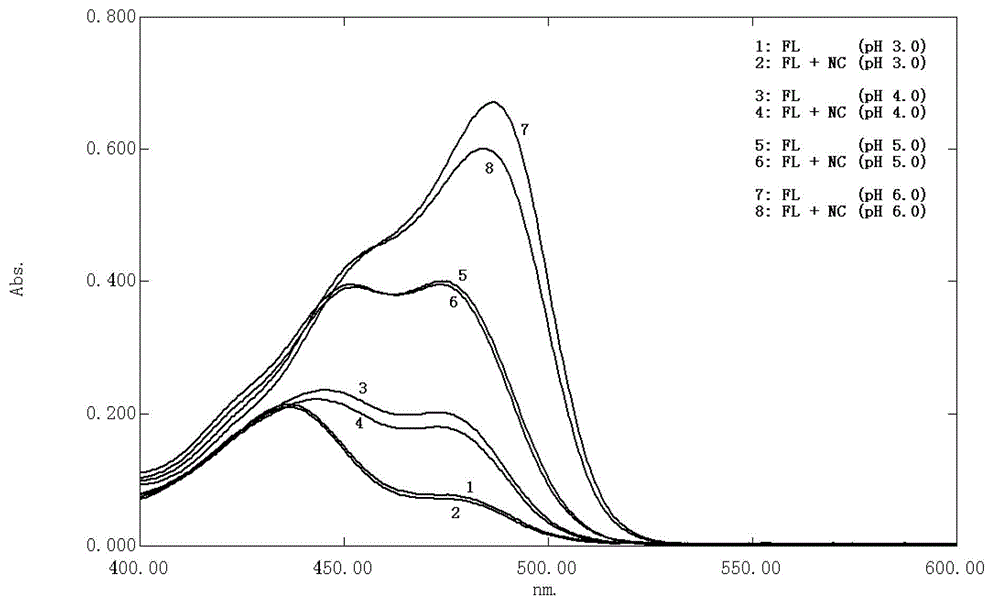
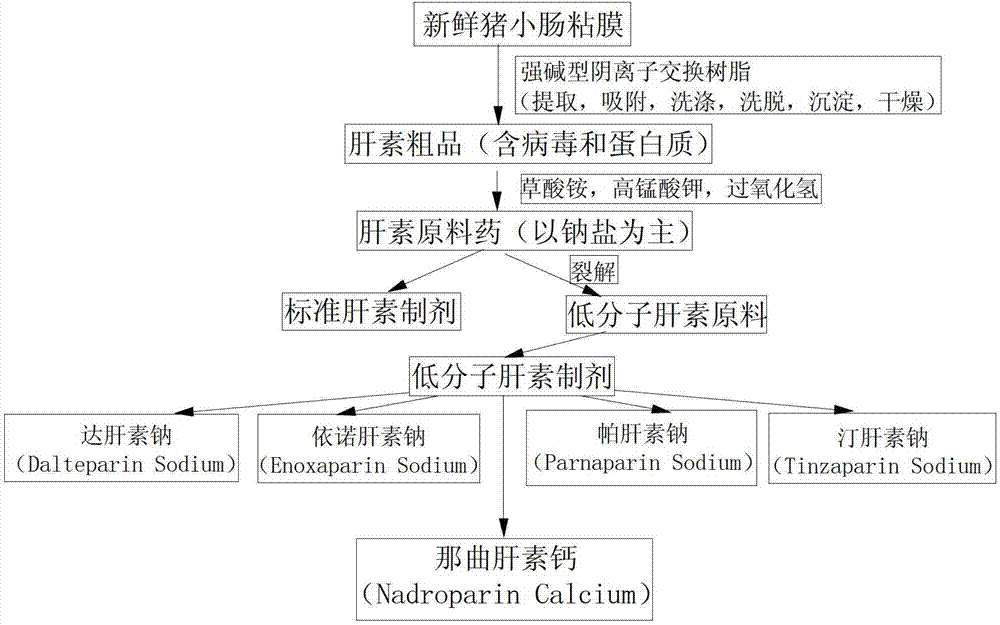
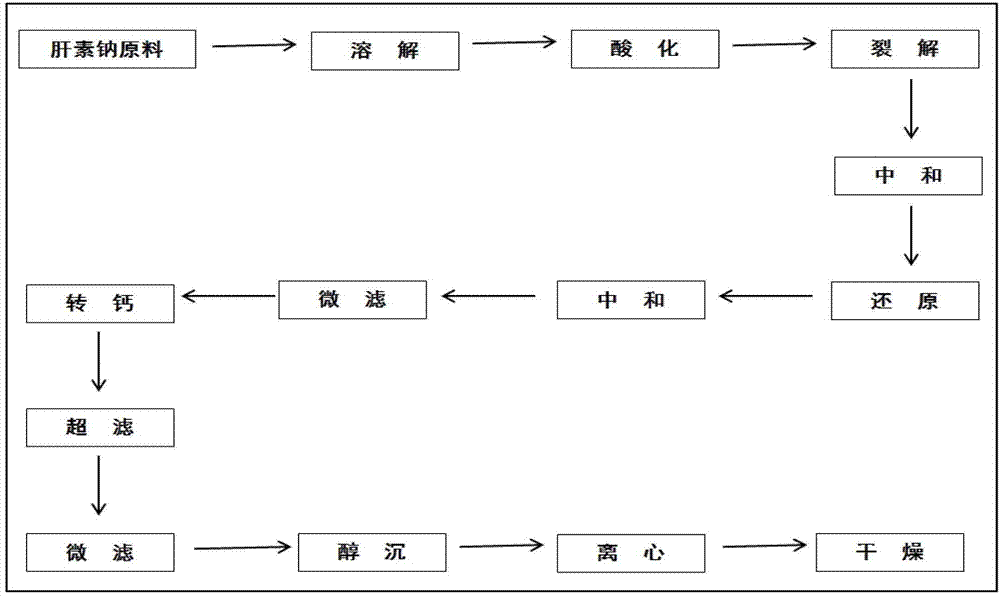
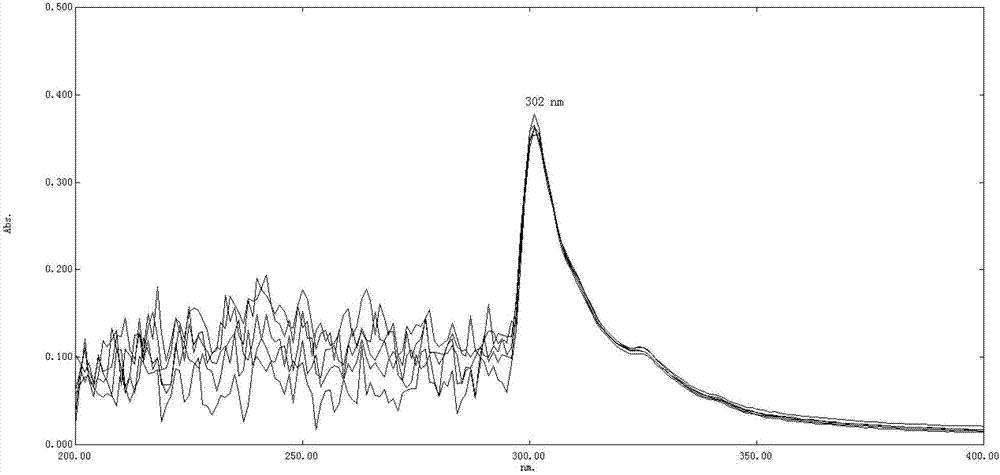
Patents
Literature
34 results about "Nadroparin calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
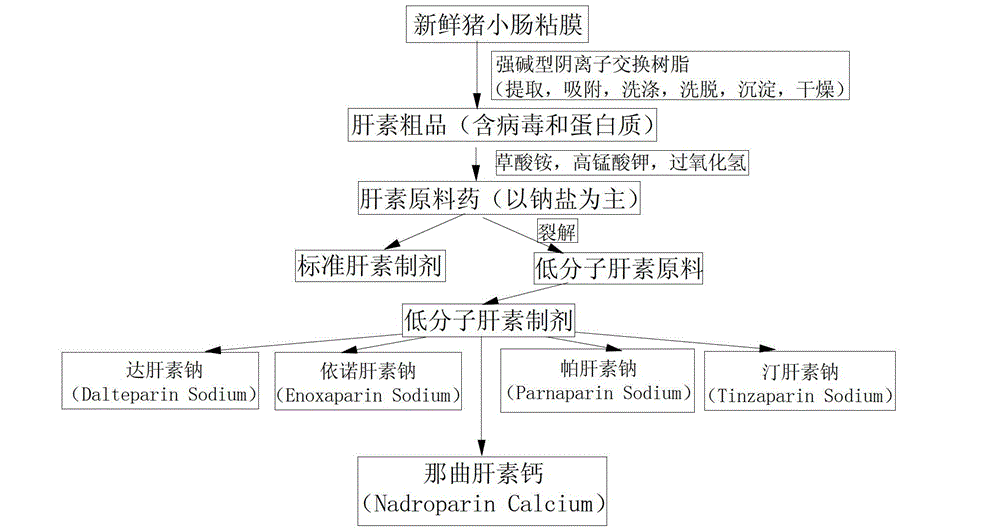
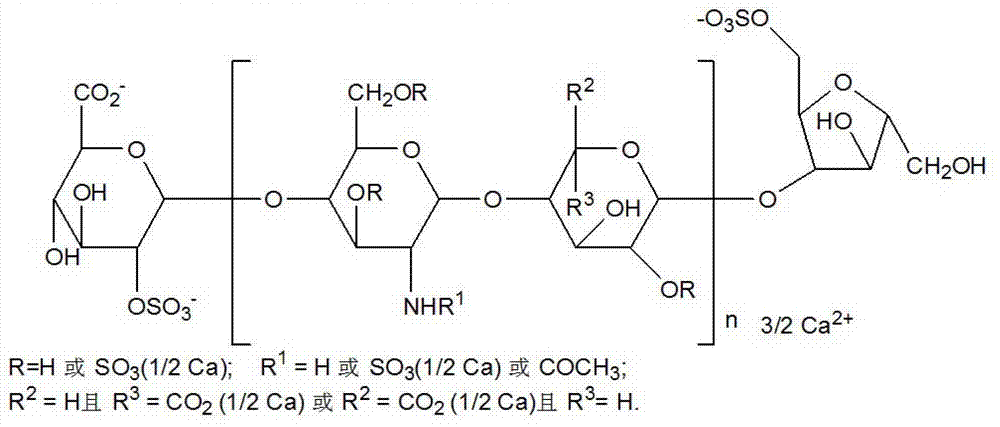
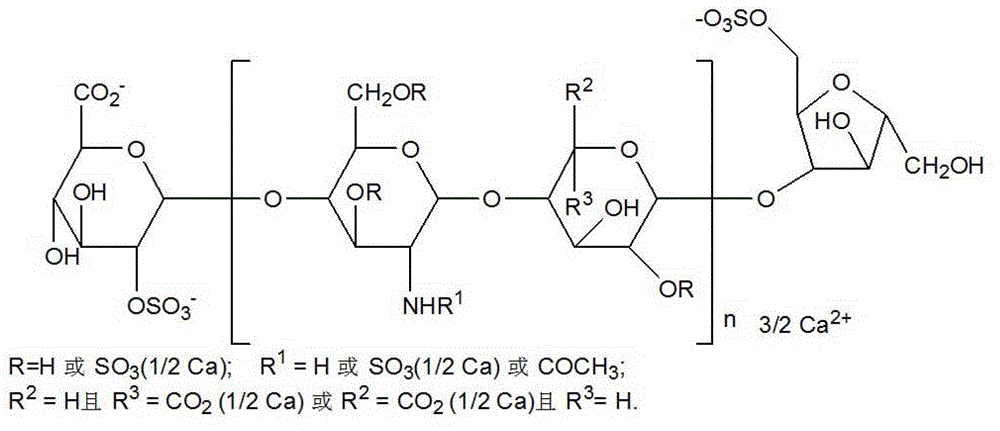
Nadroparin (trade names Fraxiparin[e], Fraxodi, among others) is an anticoagulant belonging to a class of drugs called low molecular weight heparins (LMWHs). Nadroparin was developed by Sanofi-Synthélabo.
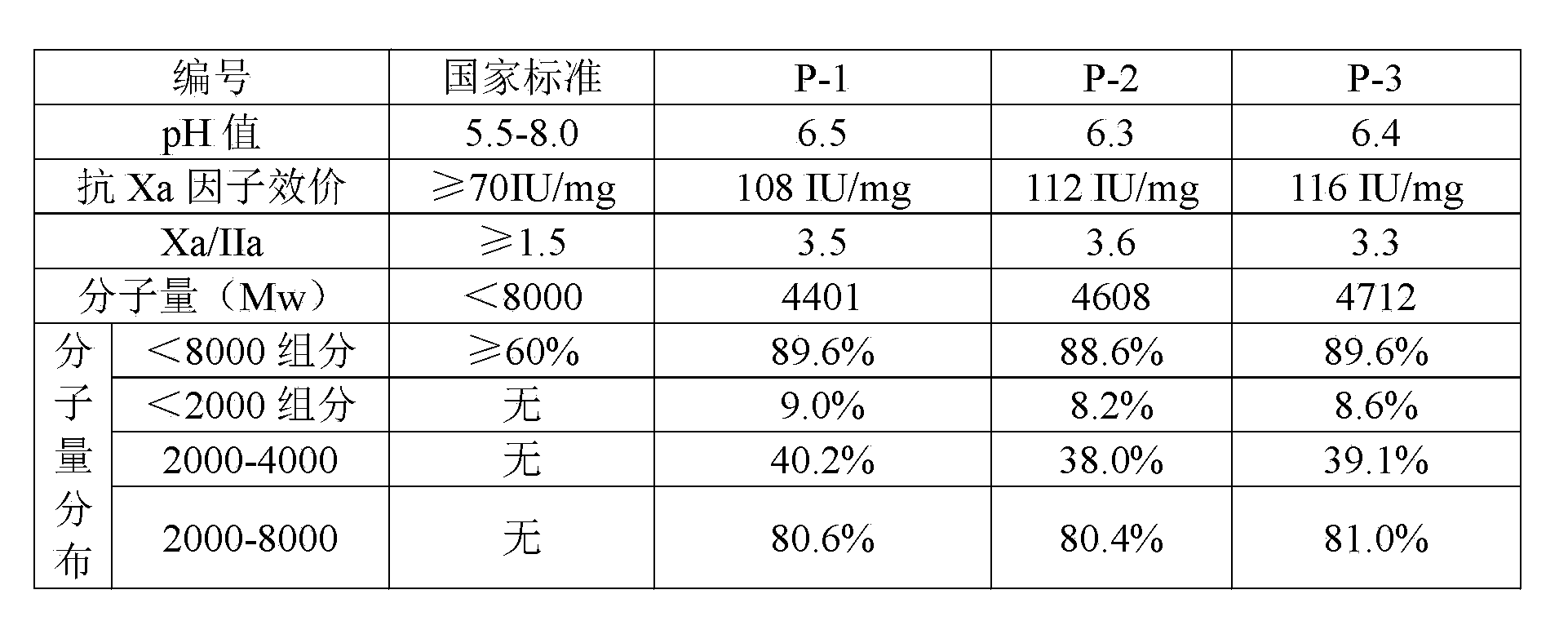
Preparation and purification process for nadroparin calcium
ActiveCN103382232AEfficient separationChange fractional precipitation methodBlood disorderExtracellular fluid disorderFractional PrecipitationDepolymerization
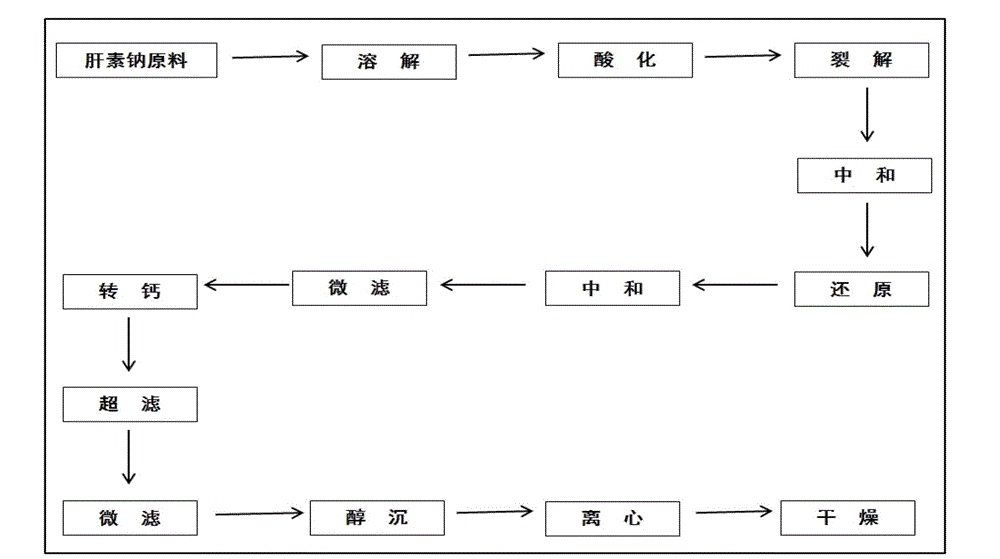
The invention relates to a preparation and purification process for nadroparin calcium. The process comprises the following steps: depolymerization; reduction; recrystallization; chromatography; and filtration. According to the invention, the process can effectively extract a fragment with a narrow molecular weight distribution range; nadroparin calcium is obtained through improvement of a preparation process for low-molecular-weight heparin calcium, an out-dated solvent fractional precipitation method is changed, conditions for anion exchange chromatography are optimized, and the fragment both meeting requirements for molecular weight of LMWH in China and according with molecular weight distribution of nadroparin calcium are obtained through gradient?elution.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
Preparation process of nadroparin calcium
ActiveCN104086673AHigh activityHighly Active Nadroparin Calcium Product, Product QualityAntipyreticAnalgesicsNitro compoundFreeze-drying
The invention relates to a preparation process of nadroparin calcium. The preparation process comprises the following steps: S1, degrading heparin sodium; S2, reducing degradation liquid; S3, performing ultraviolet radiation, namely radiating reduction liquid by using an ultraviolet lamp to remove residual nitro compounds from the reduction liquid and reduce the content of N-NO groups; S4, performing calcium conversion and concentration, namely performing ultrafiltration on an ultrafiltration membrane by using a 5% calcium chloride solution, and concentrating the reduction liquid until the solid-liquid ratio is 1:10; S5, performing anion exchange chromatography, namely performing chromatography by adopting anion exchange resin, and collecting elution liquid; S6, freeze-drying to obtain nadroparin calcium. According to the process, the molecular weight of low-molecular-weight heparin calcium is controlled through the anion exchange chromatography, so that the molecular weight can be accurately controlled within 1% and the yield can be increased to more than 50%; moreover, the automation degree is high and the operation is convenient, so that a powerful guarantee is provided for increasing the production capacity.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Nadroparin calcium preparation technology
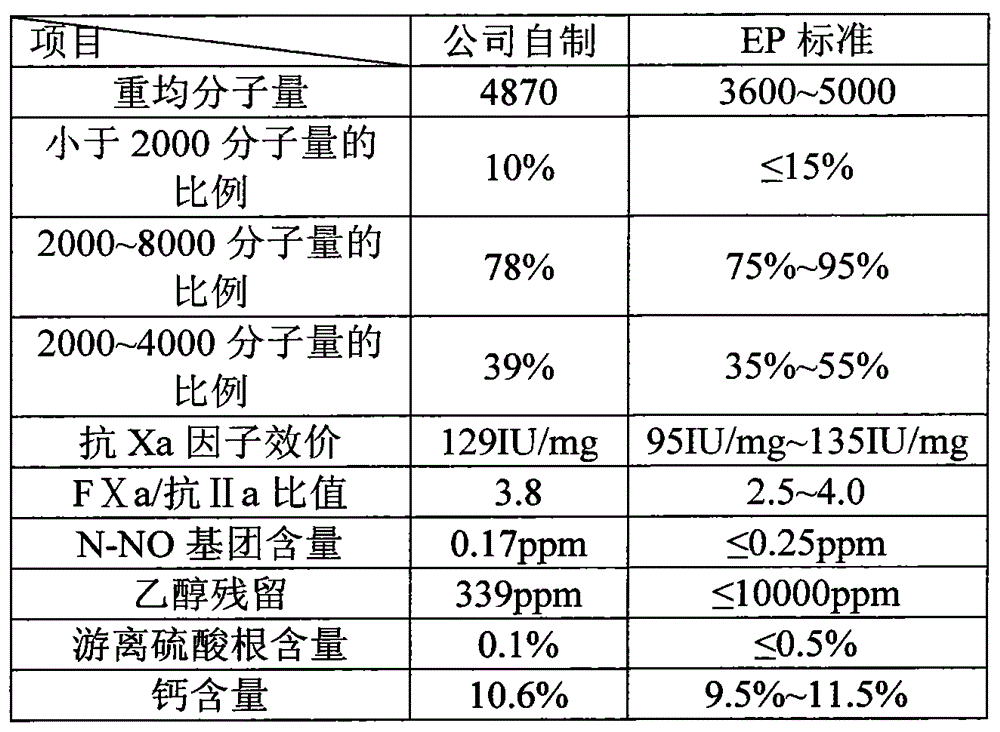
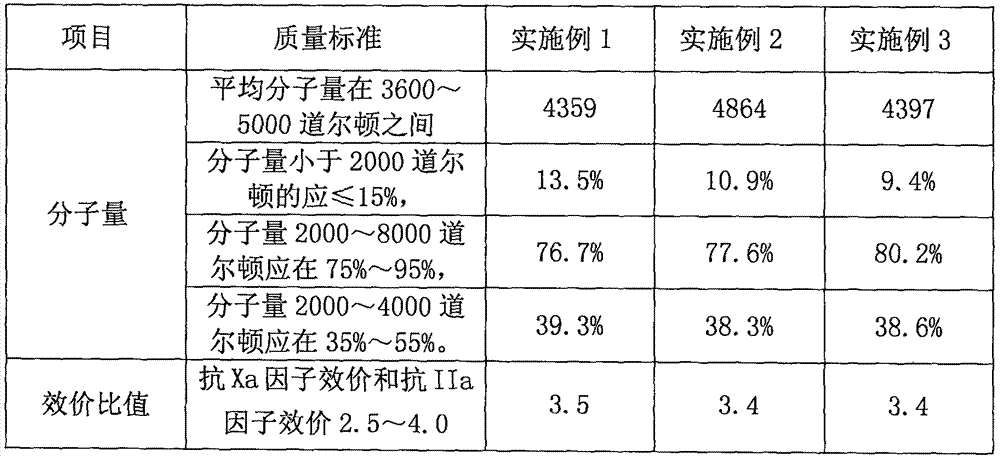
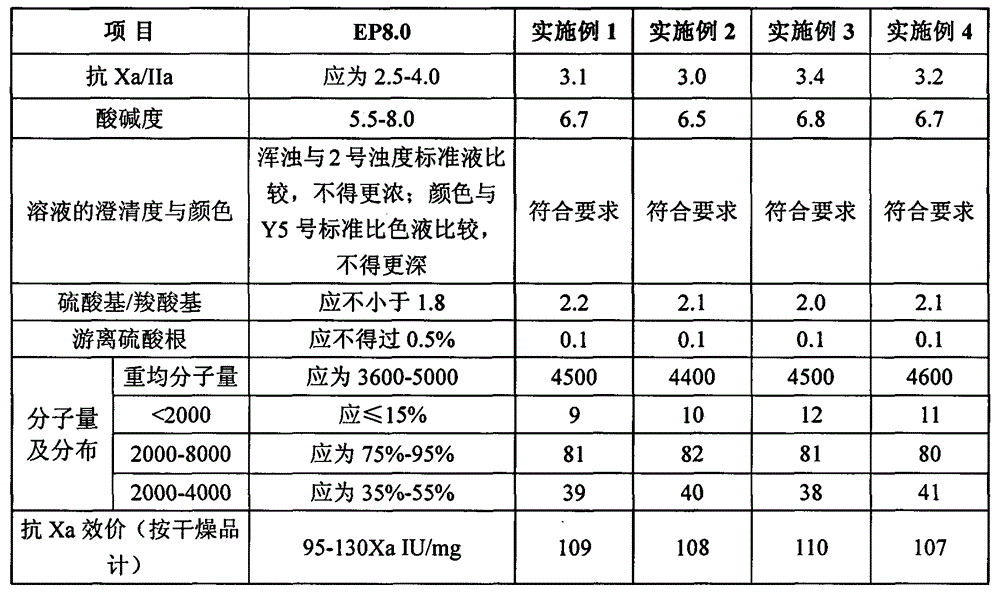
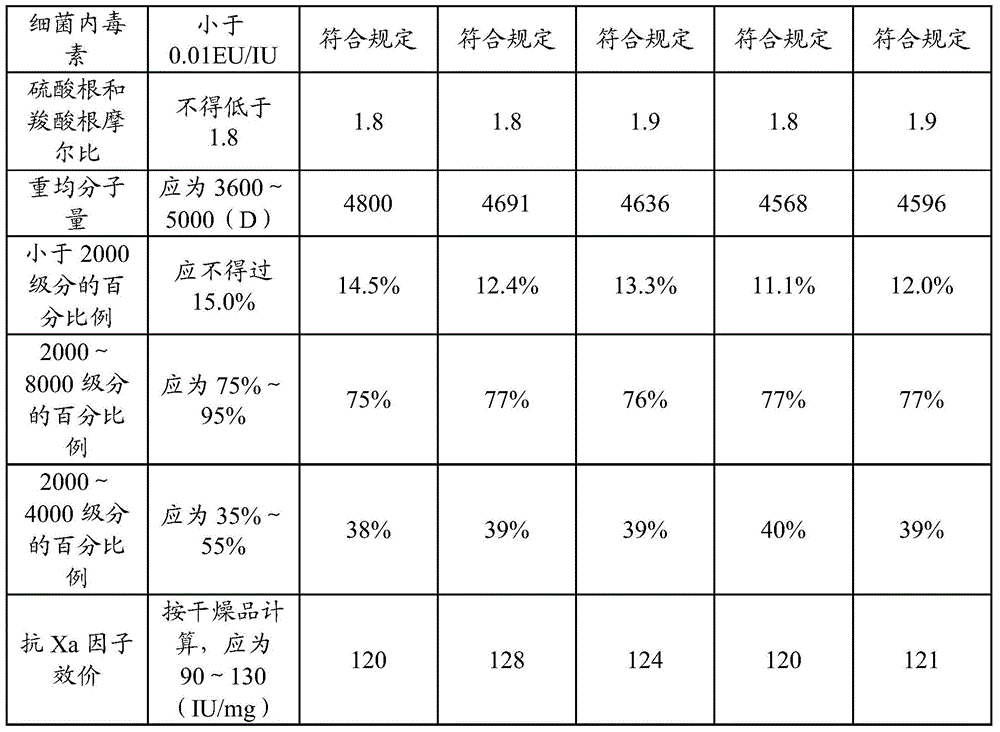
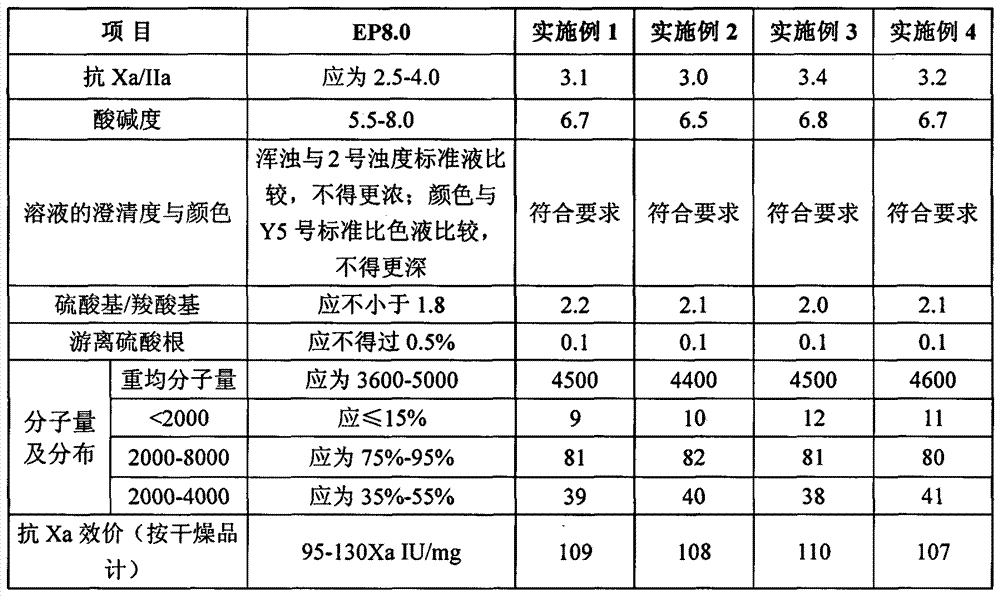
The invention discloses a nadroparin calcium preparation technology. The technology comprises the steps of degradation liquid preparation, reducing liquid preparation, precipitation, oxidation, ion exchange, crude nadroparin calcium preparation, refining, and lyophilization. The technology which adopts a chemical degradation method to produce nadroparin calcium allows the highest nadroparin calcium yield to reach 75%, and has the advantages of simplicity, abundant sources of raw materials, convenient operation, no discharge of three wastes, low cost, and stable and controllable product quality. Heparin fragments having different molecular weights are separated through membrane separation to prepare the nadroparin calcium product having an anti-Xa titer of 95-130IU / mg, an activity ratio of 2.5-4.0 and a molecular weight of 3600-5000D, and the product can reach the quality standard prescribed in European Pharmacopoeia. The product can reduce the local hematoma phenomena and obviously improve the ache condition during hypodermic injection, has the efficacies of venous thromboembolism prevention, coronary heart disease treatment, anticoagulation, antithrombotic property, antitumor, anti-inflammation, anti-allergy and blood fat adjustment, and has the advantages of substantial curative effects, wide application range and convenience for the large scale production.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Production process of nadroparin calcium with low ethanol residue
The invention discloses a production process of nadroparin calcium with low ethanol residue. The process comprises the following steps of: performing cracking, reduction, ultrafiltration and concentration on heparin sodium which is taken as a raw material to obtain a solution of the nadroparin calcium, and then regulating the concentration of the solution of the nadroparin calcium, the using quantity of anhydrous ethanol during an alcohol precipitation process, the using quantity of the anhydrous ethanol during a pulping process and the temperature during vacuum drying so as to finally obtain a finished product of the nadroparin calcium. According to the production process of the nadroparin calcium with the low ethanol residue, disclosed by the invention, the problem of the ethanol residue during the production process of the nadroparin calcium is solved, the ethanol residue in the nadroparin calcium is not more than 0.5% and in line with the requirements of European Pharmacopoeia EP7.0, the use of freeze-drying equipment is avoided, and the production process has the advantages of low price of the equipment, low operation energy consumption, convenience in maintenance, small floor area, easiness in production amplification, low production cost, stable process and the like.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Combined preparation method of dalteparin sodium and nadroparin calcium
Common sodium heparin is taken as a raw material to be fed once, and low molecular heparin obtained after degradation of the common sodium heparin is utilized for preparing two products including dalteparin sodium and nadroparin calcium by virtue of an ethanol fractional precipitation method.
Owner:YANTAI DONGCHENG PHARMA GRP
Method for producing nadroparin calcium by using crude sodium heparin products
ActiveCN104558250ASimple manufacturing processReduce multiple oxidationDepolymerizationUltrafiltration
The invention discloses a method for producing nadroparin calcium by using crude sodium heparin products. The method is implemented by taking crude sodium heparin products as a raw material through the steps of pretreating the crude sodium heparin products by using a salt hydrolysis process; sequentially carrying out oxidation, ion exchange resin adsorption, washing and elution on the obtained object; sequentially carrying out ultrafiltration and freeze-drying on the obtained product so as to obtain a fine sodium heparin product; and sequentially carrying out depolymerization, reduction, grading-alcoholic precipitation, calcium transfer, and freeze-drying treatment on the fine sodium heparin product, so that nadroparin calcium is obtained. The method disclosed by the invention has the advantages that the quality of nadroparin calcium is controlled from the aspects of source and process, the production cycle is short, the energy consumption of production is low, and the quality of products is high, therefore, the method is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
High-purity nadroparin calcium
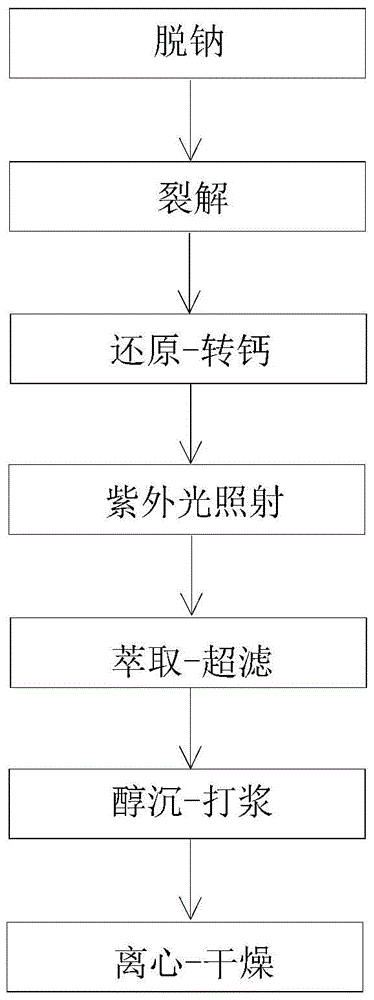
ActiveCN104804110ANarrow distributionReduce the burden onBlood disorderExtracellular fluid disorderForeign matterFiltration
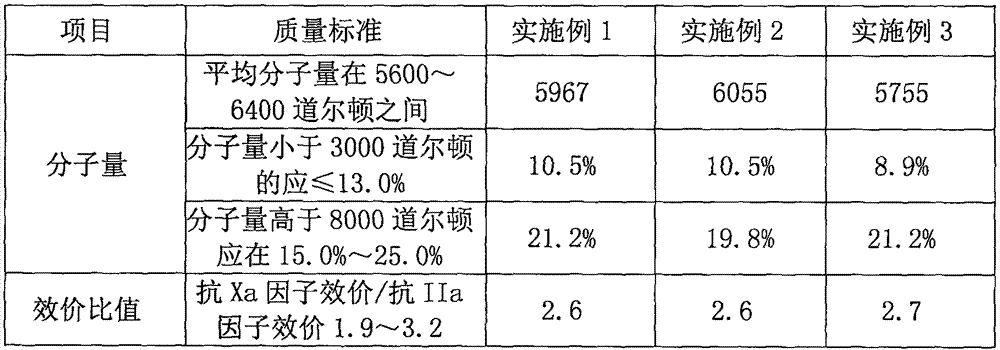
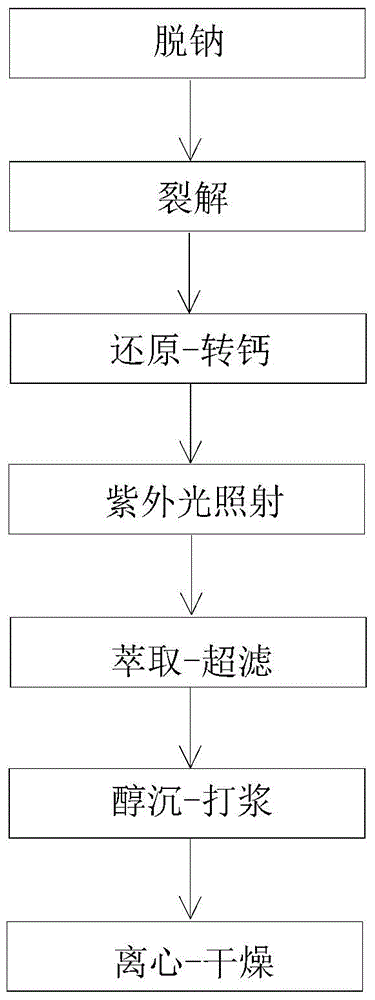
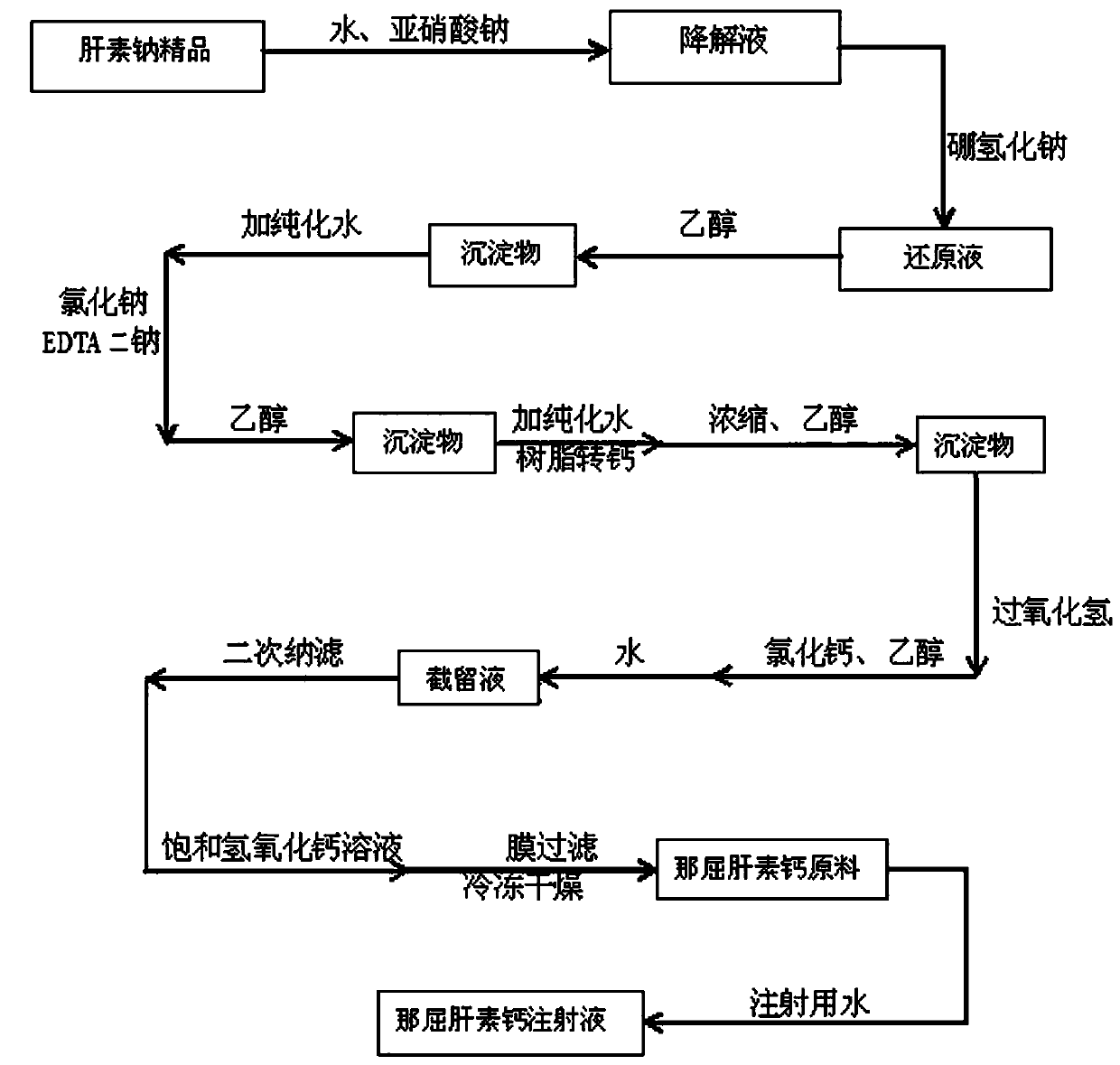
The invention relates to the technical field of heparin medicines, in particular to high-purity nadroparin calcium. The high-purity nadroparin calcium is prepared by the steps of sodium removal, splitting, restoration-calcium-conversion, ultraviolet light irradiation, extraction-ultra-filtration, alcohol precipitation-pulping and centrifugation-drying; strong acid cation exchange resin is added into a heparin sodium solution to enable the heparin sodium solution to reach a certain pH range as well as to remove about 1 / 3 sodium ions from the heparin sodium solution through ion exchange and filtration so as to reduce the load of calcium-conversion in the late period; calcium oxide is used to replace the sodium hydroxide for neutralizing the reaction liquid to reduce the intake amount of the sodium ions in the reaction liquid; sodium borohydride and excessive calcium chloride are both added into the reaction liquid and are stirred for a proper duration for achieving a better calcium-conversion effect; the primary feed liquid is irradiated by the ultraviolet light, so that the vast majority of N-NO foreign matters are removed to enable the content of the N-NO to meet the most strict EP (European pharmacopoeia) demand.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Method for producing nadroparin calcium from heparin sodium crude product
The invention discloses a method for producing nadroparin calcium from a heparin sodium crude product. The method comprises the following steps: dissolving the raw material crude product heparin sodium in water, precipitating by centrifugation and secondary salting-out to remove impurities in the heparin sodium crude product, carrying out nitrous degradation and reduction to obtain low-molecular heparin containing free sulfates, adding barium chloride to remove the free sulfates generated by degradation, and using an anion exchange resin and adjusting to appropriate parameters to carry out calcium conversion and final impurity removal; and finally, carrying out oxidation decolorization, sterile filtration and dehydration to obtain the nadroparin calcium raw material. By using the crude product heparin sodium as the raw material, the production cost is lowered; the technical steps are simplified to enhance the yield of the nadroparin calcium and reduce the titer loss of the nadroparin calcium; and the method can ensure the safety of the product, and is convenient for industrialized mass production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Production method for nadroparin calcium
ActiveCN104072639AProduction process optimization and improvementRealize industrial productionEthanol precipitationIon-exchange resin
The invention discloses a production method for nadroparin calcium. Accoding to the production method, heparin sodium is adopted as raw material and subjected to degradation, deoxidation, ethanol precipitation, ion exchange resin calcium conversion, oxidation, filtration, precipitation and dehydration to obtain nadroparin calcium; the one-pot boiling technology is realized through oxidation, sterile filtration, precipitation and dehydration and the simplified operation is realized; compared with the disclosed methods, steps adopted in the heparin sodium preparation technology are fewer, the operation is simpler and more convenient, the calcium conversion is more complete and the yield is higher.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
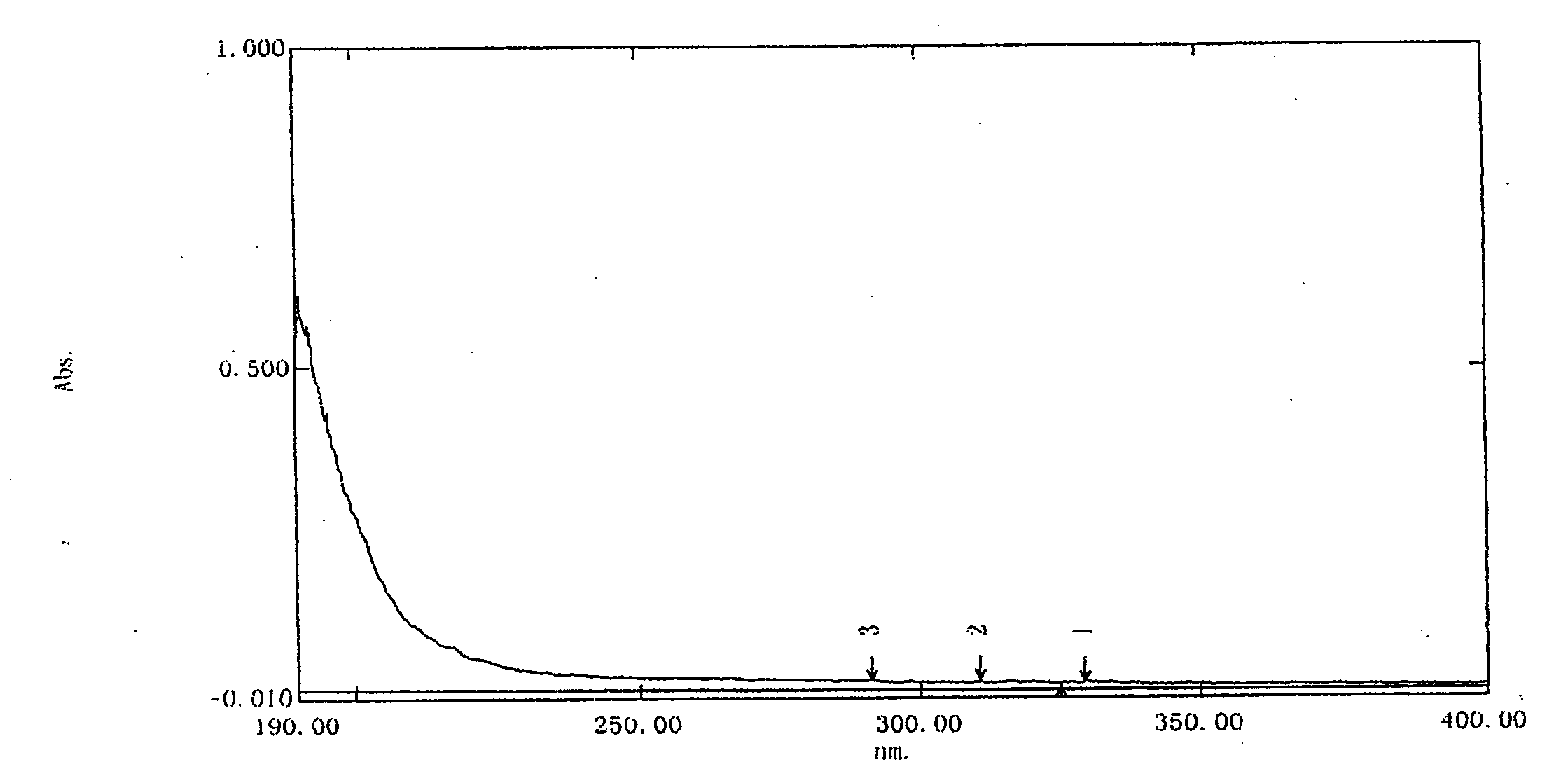
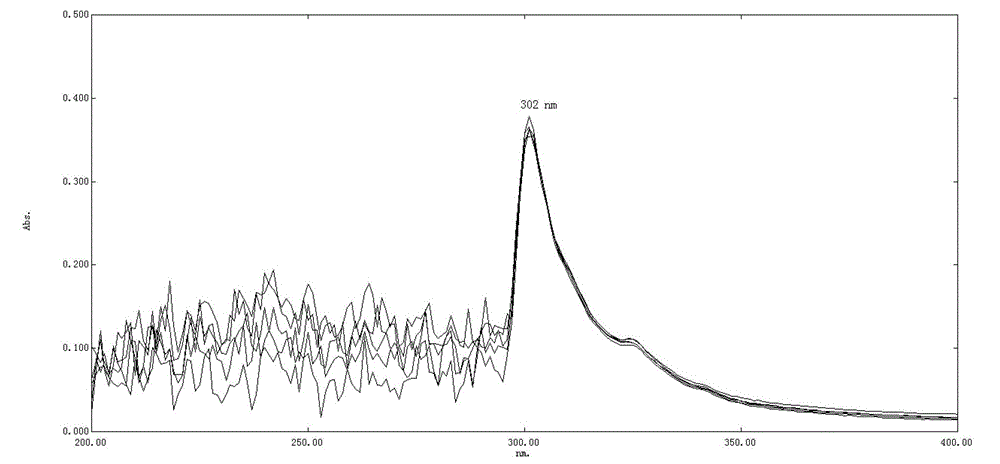
Solution concentration of nadroparin calcium determined by phenanthroline-zinc sulfate ultraviolet spectroscopy
ActiveCN103149170ARapid Quantitative DetectionImprove anti-interference abilityColor/spectral properties measurementsAnti jammingUltraviolet
The invention discloses solution concentration of nadroparin calcium determined by phenanthroline-zinc sulfate ultraviolet spectroscopy. Under the environment of buffered solution, Ultraviolet absorbance degrees of mixed solution which includes Zn2+ions, phenanthroline and the nadroparin calcium are determined, and then the concentration of the nadroparin calcium is determined by quantity. According to the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy, a phenanthroline-zinc sulfate ultraviolet spectroscopy test system is applied, quick quantitative determination of the solution concentration of the nadroparin calcium can be achieved. The linear range of an analytical method of the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy is 1.184%-16.33%, meanwhile, the analytical method of the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy has good anti-jamming capability and can be applied in the practical production process of nadroparin calcium medicines and capable of carrying out concentration determination to intermediate product solution or finished product solution.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Method for preparing nadroparin calcium
InactiveCN109748984AImprovement of yellowish colorSolve the disadvantages of yellowing colorFiltrationUltrafiltration
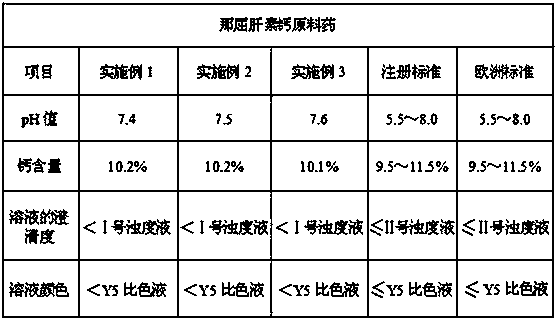
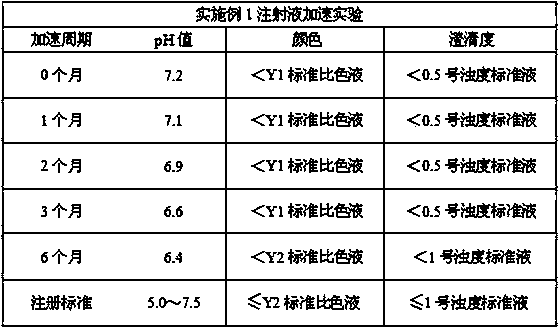
The invention discloses a method for preparing nadroparin calcium. The method for preparing the nadroparin calcium includes the steps of adopting heparin sodium as the raw material to conduct variousprocesses such as degradation, reduction, removal of metal ions, calcium transfer, nanofiltration, oxidation, ultrafiltration, nanofiltration, membrane filtration and freeze drying to prepare the nadroparin calcium. The method for preparing the nadroparin calcium has the advantages that with the raw material of the nadroparin calcium filled into injection, the yellowing of the solution is improvedsignificantly, the risks of turbidity and pH (potential of hydrogen) reduction in the nadroparin calcium injection are reduced, and the controllability of the pH of the injection prepared from the raw material of the nadroparin calcium is guaranteed; with the raw material going through 6-month accelerated stability test and 24-month long-term stability test after the prepared raw material is filled into the injection, the stability of the product is good, the test results are in accordance with the regulations and standards, the safety of the product, high biological activity and good productquality are ensured in clinical use, and the risk of adverse reactions is reduced; the prepared nadroparin calcium-filled injection has good stability in various aspects such as color, clarity and pHvalue; the method of significant social and economic benefits is simple and suitable for commercial production.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method for detecting nadroparin calcium product
ActiveCN103398960AAvoid interferenceThe instrument is simpleColor/spectral properties measurementsNitrite ionAbsorbance
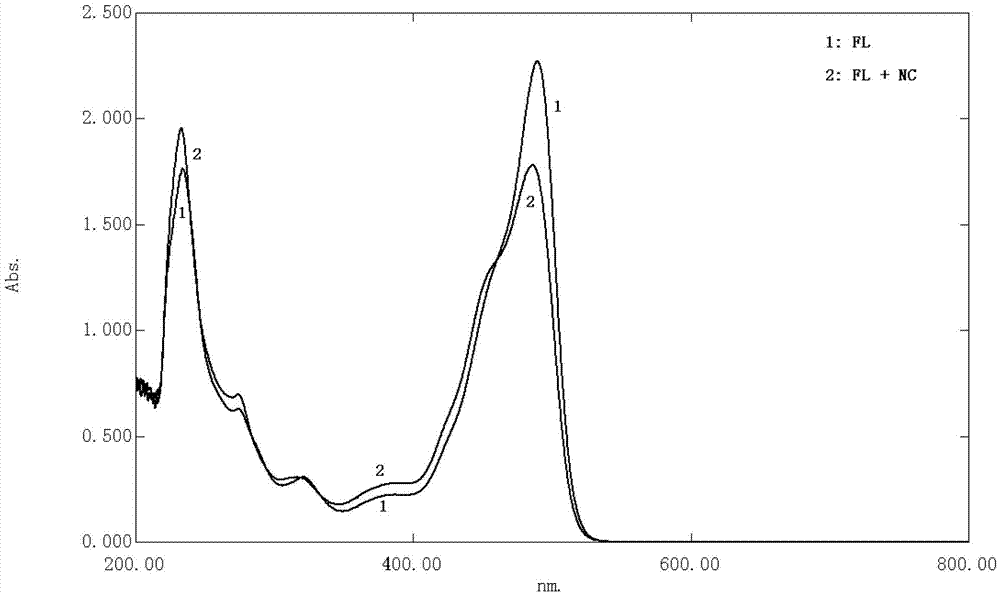
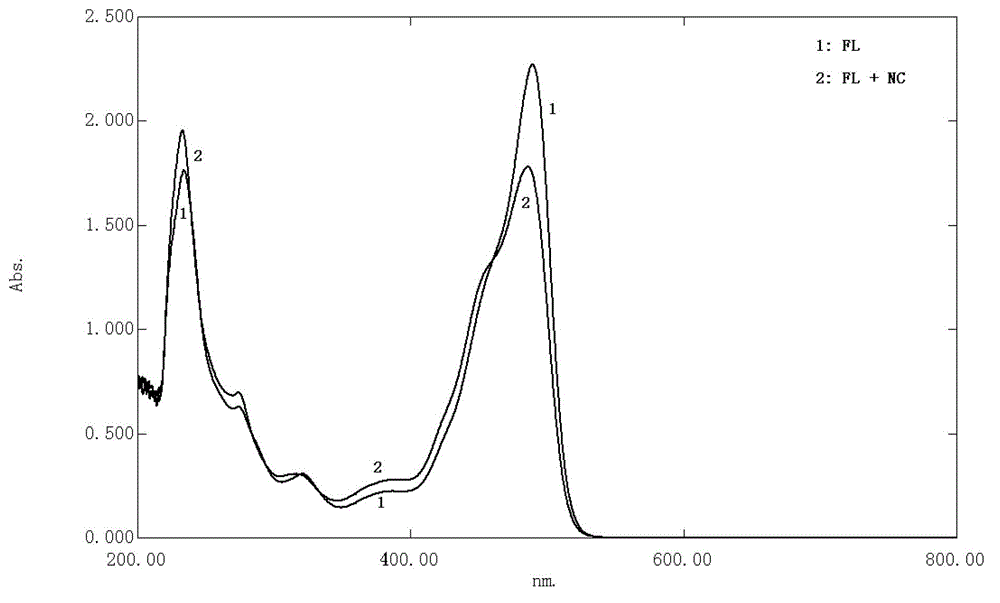
The invention relates to the technical field of ultraviolet-visible spectrophotometric method detection and particularly relates to a method for rapidly detecting a nadroparin calcium product. The method comprises the step of detecting the concentration of nadroparin calcium in a solution through detecting the absorbance of a mixed solution in a range of 200nm-800nm by a spectrophotometric method, wherein the mixed solution contains fluorescein, the nadroparin calcium and a buffering solution; preferably, the mixed solution is formed by mixing an ethanol solution of the fluorescein, a nadroparin calcium solution, a sodium sulfate solution and the buffering solution. According to the method disclosed by the invention, an applied instrument is simple and the operation is simple and convenient; the concentration of the nadroparin calcium solution can be rapidly determined. The detection method provided by the invention is strong in anti-interference capability and strong in selectivity, and can be used for monitoring whether the contents of nitrite ions and calcium ions in the nadroparin calcium solution seriously exceed the standard or not. According to the detection method provided by the invention, a linear range is 3.0*10<-3>mol / L-4.5*10<-2>mol / L and a detection limit is 1.0*10<-3>mol / L; the detection method is completely applicable to the detection of a nadroparin calcium middle product and a nadroparin calcium final product and can be used for effectively monitoring a medicine production process of the nadroparin calcium.
Owner:广东三生制药有限公司
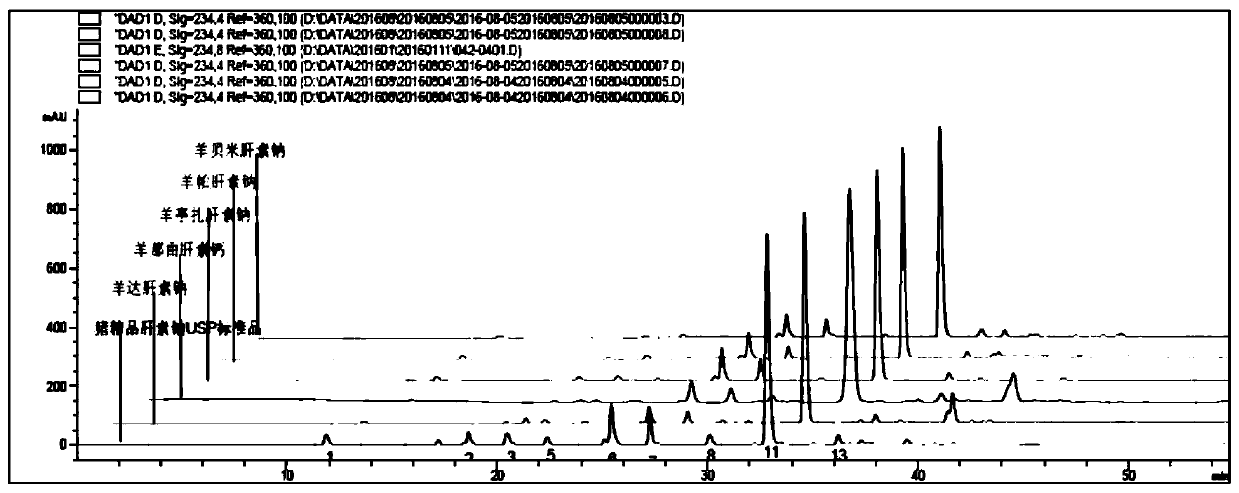
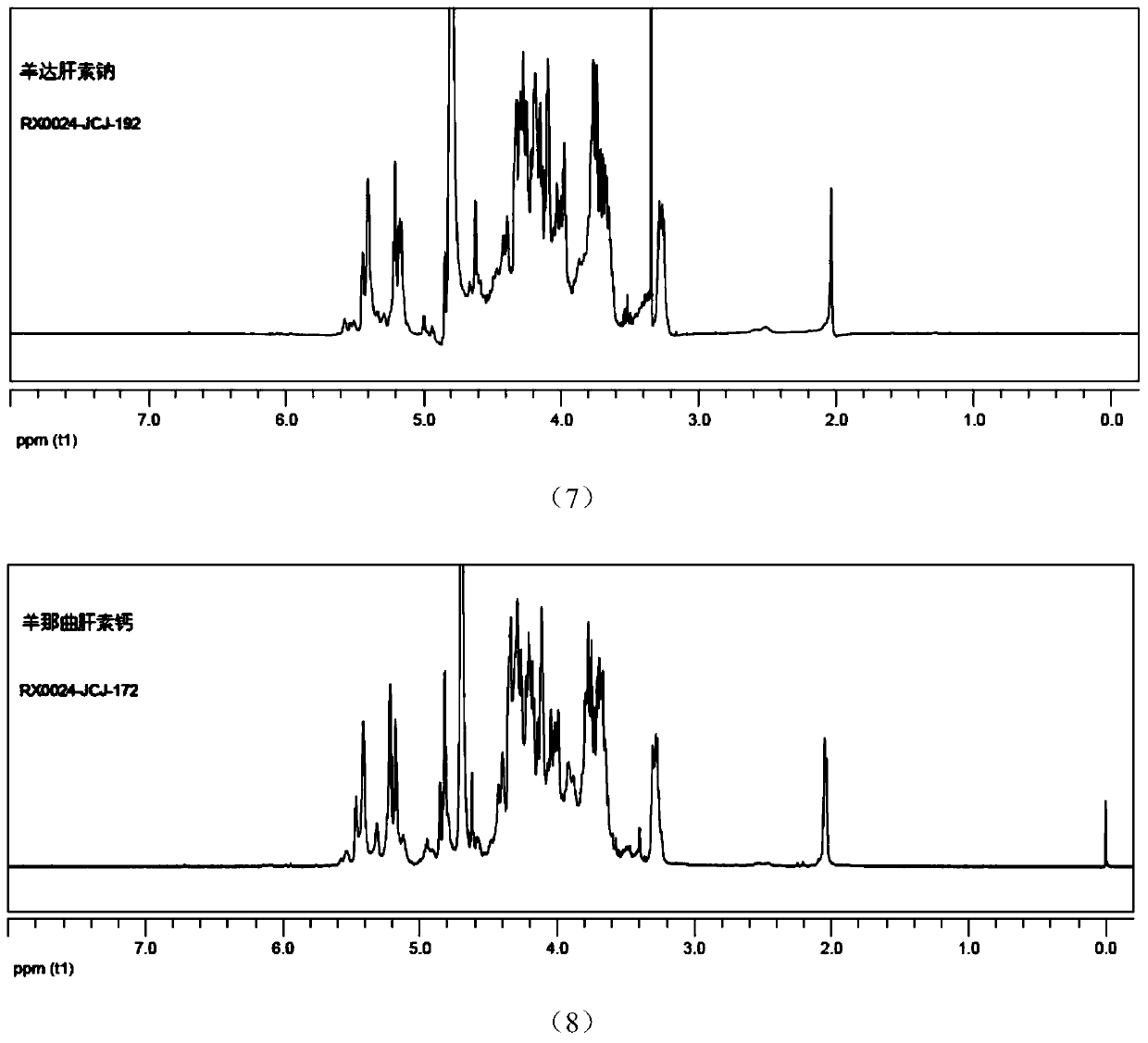
Sheep-sourced low molecular heparins as well as preparation method and application thereof
ActiveCN107759712ARich sourcesAbundant productionOrganic active ingredientsBlood disorderChemical structureComputational chemistry
The invention discloses sheep-sourced low molecular heparins which comprise ovine dalteparin sodium, ovine nadroparin calcium, ovine tinzaparin sodium, ovine parnaparin sodium and ovine bemiparin sodium. All the sheep-sourced low molecular heparins have common provenance characteristics; from chemical structures, the content of main disaccharide deltaUA2S-GlcNS6S(deltaIS) is 66 to 74%. The physical and chemical properties of the sheep-sourced low molecular heparins are similar to the physical and chemical properties of pig-sourced low molecular heparins, and the biological activities are close, so that sources of low molecular heparin type drugs are expanded. A preparation method of the sheep-sourced low molecular heparins disclosed by the invention is simple, feasible and stable in process; obtained products are refined, and can completely meet requirements of each pharmacopeia on conventional low molecular heparins. The sheep-sourced low molecular heparins also have the muslim whichis absent in the pig-sourced low molecular heparins, so that the sheep-sourced low molecular heparins have a giant market in general muslim people, countries and districts, and can be applied to anticoagulation, antithrombotic, anti-inflammatory, anti-cancer and muslim drugs.
Owner:SUZHOU RONGXI BIOTECH CO LTD +1
Drying method of nadroparin calcium
InactiveCN106699928AMeet the quality standard requirementsImprove solubilitySolubilityWork in process
The invention belongs to the field of biological medicine, in particular, relates to a spray drying process of glycosaminoglycans, and particularly, relates to a spray drying process of nadroparin calcium. The spray drying method is adopted; spray drying is a drying technology comprising the steps of dispersing a raw material into fog droplets by a liquid atomizer, making hot air (or other gases) direct contact with the fog droplets, carrying out heat exchange, and rapidly evaporating a solvent in the fog droplets, to obtain a powder or fine granular finished product or semi-finished product. The technology has the advantages of short drying time, less damage to active ingredients, uniform texture and good solubility. The invention aims to search the preparation method suitable for industrial production of nadroparin calcium under conditions of different drying ways of nadroparin calcium, and the high-quality nadroparin calcium product can be simply and efficiently obtained with low cost.
Owner:YANTAI DONGCHENG PHARMA GRP
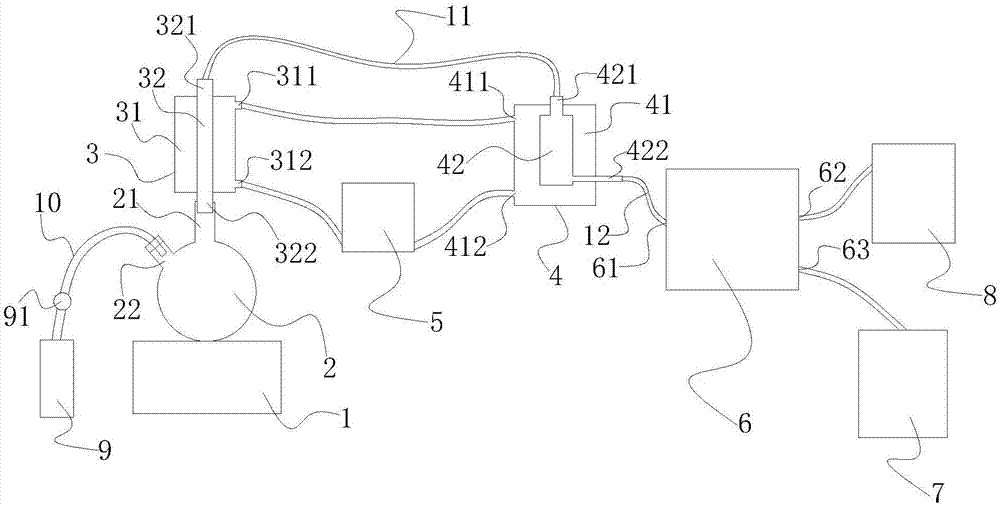
System and method for detecting N-NO in nadroparin calcium by chemical extraction-thermal energy analysis method
InactiveCN107328763AAccurate detectionSuitable for testingChemiluminescene/bioluminescenceThermal energyPeristaltic pump
The invention relates to a system and a method for detecting N-NO in nadroparin calcium by a chemical extraction-thermal energy analysis (TEA) method. The system comprises a TEA analyzer, a first-grade condensing device, a second-grade condensing device, a peristaltic pump, a heater, a reactor, an oxygen gas source, a vacuum pump and a nitrogen gas source. The method comprises the following steps: S1: preparing a solution; S2: carrying out instrument regulation preparation and the like; S3: opening two grades of condensing devices to enable the two grades of condensing devices to start condensing medium circulation; S4: adding one part of a reaction system from a sample inlet of the reactor and regulating a nitrogen gas flow speed and the like; S5: adding a standard product solution and a test sample solution and recording a chromatogram; calculating to obtain a result. The system provided by the invention is ingenious and simple and can be completely suitable for the detection of NO in the nadroparin calcium. The method provided by the invention has a more accurate result, lower detection limit and a more rapid detection speed and is simpler. By adopting the method provided by the invention, a procedure is greatly simplified; the method has extremely high sensitivity and high selectivity on a nitrogen-containing compound and is a detection method with extremely strong specificity.
Owner:CHANGZHOU QIANHONG BIOPHARMA
A kind of high-purity nadroparin calcium
ActiveCN104804110BNarrow distributionReduce the burden onBlood disorderExtracellular fluid disorderForeign matterFiltration
The invention relates to the technical field of heparin medicines, in particular to high-purity nadroparin calcium. The high-purity nadroparin calcium is prepared by the steps of sodium removal, splitting, restoration-calcium-conversion, ultraviolet light irradiation, extraction-ultra-filtration, alcohol precipitation-pulping and centrifugation-drying; strong acid cation exchange resin is added into a heparin sodium solution to enable the heparin sodium solution to reach a certain pH range as well as to remove about 1 / 3 sodium ions from the heparin sodium solution through ion exchange and filtration so as to reduce the load of calcium-conversion in the late period; calcium oxide is used to replace the sodium hydroxide for neutralizing the reaction liquid to reduce the intake amount of the sodium ions in the reaction liquid; sodium borohydride and excessive calcium chloride are both added into the reaction liquid and are stirred for a proper duration for achieving a better calcium-conversion effect; the primary feed liquid is irradiated by the ultraviolet light, so that the vast majority of N-NO foreign matters are removed to enable the content of the N-NO to meet the most strict EP (European pharmacopoeia) demand.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
A kind of preparation technology of nadroparin calcium
The invention relates to a preparation process of nadroparin calcium, comprising the following steps: S1 degradation of heparin sodium; S2 reduction of degradation solution; S3 ultraviolet irradiation: using an ultraviolet lamp to irradiate the reduction solution to eliminate residual nitroso groups in the reduction solution compound, reducing the N-NO group content; S4 calcium transfer and concentration: use 5% calcium chloride solution to ultrafilter on the ultrafiltration membrane, and concentrate the reducing solution to a concentration of 1:10 in the solid-liquid ratio; S5 anion exchange layer Analysis: use anion exchange resin for chromatography, and collect the eluate; S6 freeze-drying to obtain the nadroparin calcium. The process uses anion exchange chromatography to control the molecular weight of low-molecular-weight heparin calcium, so that the molecular weight can be accurately controlled within 1%, and the yield can be increased to more than 50%, and the degree of automation is high, and the operation is convenient, which provides a powerful way to increase production capacity. Assure.
Owner:CHANGZHOU QIANHONG BIOPHARMA
A kind of production method of nadroparin calcium
ActiveCN103275246BEfficient degradationAvoid the tedious process of repeated impurity removalFreeze-dryingUltrafiltration
The invention discloses a production method of nadroparin calcium and belongs to the field of biomedicine. According to the method, crude heparin sodium is taken as raw material. Basing on enzymolysis and improved nitrite degradation method, nadroparin calcium high-quality product with particular average molecular weight (3800 to 5000Da) is prepared by enzymolysis, oxidative decolorization, ultrafiltration and impurity removal, ethanol precipitation and impurity removal, degradation, reduction, ultraviolet radiation, oxidation, Calcium replacement, nanofiltration, Ultrafiltration and refining, freeze-drying and other steps. The method provided by the invention has advantages of simple preparation technology, high bioavailability of the product, long half life in vivo, small hemorrhagic tendency and the like.
Owner:山东辰龙药业有限公司
Production method for nadroparin calcium
ActiveCN104072639BProduction process optimization and improvementRealize industrial productionEthanol precipitationIon-exchange resin
The invention discloses a production method for nadroparin calcium. Accoding to the production method, heparin sodium is adopted as raw material and subjected to degradation, deoxidation, ethanol precipitation, ion exchange resin calcium conversion, oxidation, filtration, precipitation and dehydration to obtain nadroparin calcium; the one-pot boiling technology is realized through oxidation, sterile filtration, precipitation and dehydration and the simplified operation is realized; compared with the disclosed methods, steps adopted in the heparin sodium preparation technology are fewer, the operation is simpler and more convenient, the calcium conversion is more complete and the yield is higher.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Method for preparing nadroparin calcium and dalteparin sodium
ActiveCN112830980AChemical industryChemical/physical/physico-chemical microreactorsUltrafiltrationPhysical chemistry
The invention belongs to the field of preparation of low-molecular-weight heparin, and relates to a method for preparing nadroparin calcium and dalteparin sodium. Specifically, the invention relates to a method for preparing low-molecular-weight heparin, which comprises the steps of heparin sodium cracking, reduction, neutralization and ultrafiltration in a microchannel reactor, and the low-molecular-weight heparin is selected from nadroparin calcium and dalteparin sodium. According to the method, the selectivity of heparin sodium cracking reaction is improved, and the probability of repeated cracking is reduced, so that the increase of micromolecular heparin is avoided, the load of later ultrafiltration molecular weight screening is reduced, and the yield of the product is improved.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Sheep-derived low-molecular-weight heparin and its preparation method and application
ActiveCN107759712BRich sourcesAbundant productionOrganic active ingredientsBlood disorderDrug biological activitySodium heparin
The invention discloses low-molecular-weight heparin derived from sheep, including sheep dateparin sodium, sheep nadroparin calcium, sheep tinzaparin sodium, sheep betaparin sodium and sheep bemiparin sodium. The low molecular weight heparins of sheep origin above all have common provenance characteristics. In terms of chemical structure, the content of the main disaccharide ΔUA2S‑GlcNS6S (ΔIS) is between 66%‑74%. Sheep low-molecular-weight heparin and pig-derived low-molecular-weight heparin have similar physical and chemical properties and biological activities, which expands the source of low-molecular-weight heparin drugs. The preparation method of sheep low-molecular-weight heparin introduced by the invention is simple and easy, and the process is stable. The obtained product is refined and can fully meet the requirements of various pharmacopoeias for the current low-molecular-weight heparin. Sheep low-molecular-weight heparin also has halal properties that porcine-derived low-molecular-weight heparin does not have. It has a huge market in the majority of Muslim populations, countries and regions, and can be used in anticoagulant, antithrombotic, anti-inflammatory, anticancer and halal drugs.
Owner:SUZHOU RONGXI BIOTECH CO LTD +1
Production process of nadroparin calcium with low ethanol residue
The invention discloses a production process of nadroparin calcium with low ethanol residue. The process comprises the following steps of: performing cracking, reduction, ultrafiltration and concentration on heparin sodium which is taken as a raw material to obtain a solution of the nadroparin calcium, and then regulating the concentration of the solution of the nadroparin calcium, the using quantity of anhydrous ethanol during an alcohol precipitation process, the using quantity of the anhydrous ethanol during a pulping process and the temperature during vacuum drying so as to finally obtain a finished product of the nadroparin calcium. According to the production process of the nadroparin calcium with the low ethanol residue, disclosed by the invention, the problem of the ethanol residue during the production process of the nadroparin calcium is solved, the ethanol residue in the nadroparin calcium is not more than 0.5% and in line with the requirements of European Pharmacopoeia EP7.0, the use of freeze-drying equipment is avoided, and the production process has the advantages of low price of the equipment, low operation energy consumption, convenience in maintenance, small floor area, easiness in production amplification, low production cost, stable process and the like.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Preparation and purification process of nadroparin calcium
ActiveCN103382232BHas anti-factor Xa activityQuality improvementBlood disorderExtracellular fluid disorderFractional PrecipitationDepolymerization
The invention relates to a preparation and purification process for nadroparin calcium. The process comprises the following steps: depolymerization; reduction; recrystallization; chromatography; and filtration. According to the invention, the process can effectively extract a fragment with a narrow molecular weight distribution range; nadroparin calcium is obtained through improvement of a preparation process for low-molecular-weight heparin calcium, an out-dated solvent fractional precipitation method is changed, conditions for anion exchange chromatography are optimized, and the fragment both meeting requirements for molecular weight of LMWH in China and according with molecular weight distribution of nadroparin calcium are obtained through gradient?elution.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
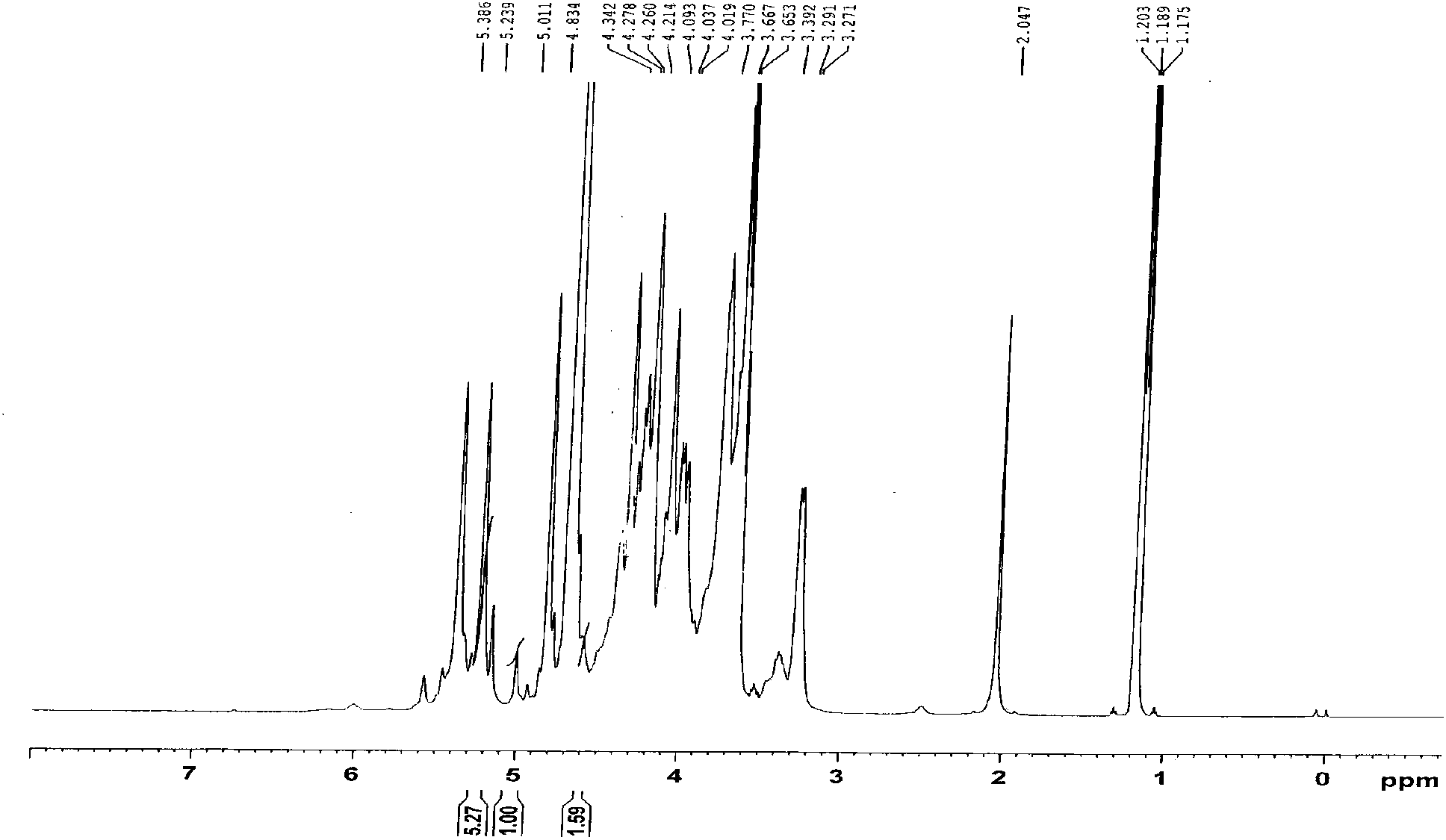
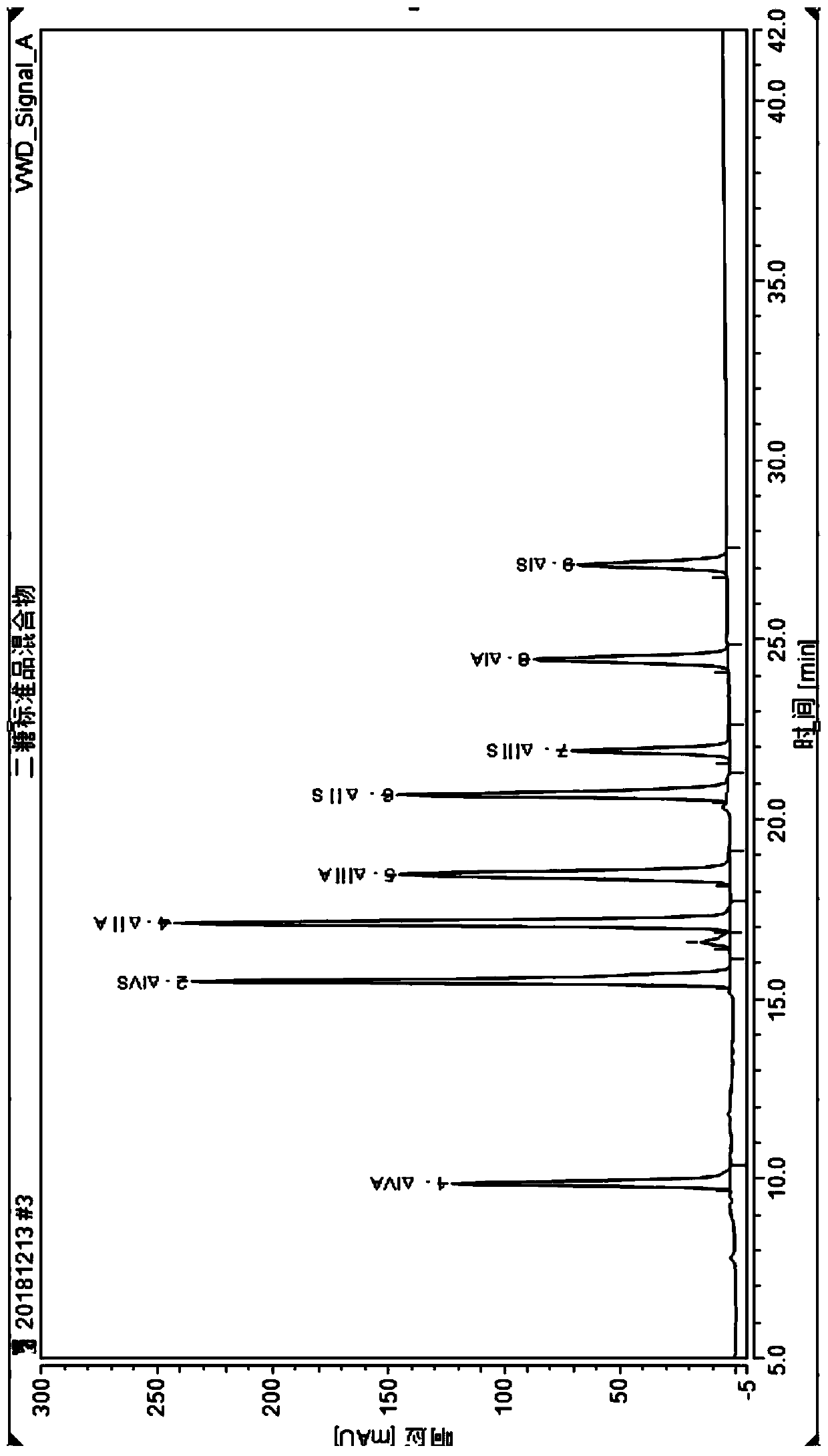
Detection method of nadroparin calcium disaccharide spectrum
The invention belongs to the technical field of raw material medicine detection, and particularly relates to a detection method of nadroparin calcium disaccharide spectrum. The detection method of thenadroparin calcium disaccharide spectrum comprises the following steps: firstly, adding mixed heparinase into a sample solution for complete enzymolysis, and then detecting an enzymolysis product byadopting an anion exchange chromatography method. According to the method, aiming at the characteristics of nadroparin calcium, the enzymolysis conditions of nadroparin calcium are deeply researched,and the disaccharide component of nadroparin calcium is detected by selecting the matched anion exchange chromatography, so that the detection result is more accurate, the sensitivity is higher, precolumn derivatization is not needed, and the operation is simpler and more convenient. The method can provide structural information for exploration of a raw material synthesis process, and can also effectively control the quality of nadroparin calcium, so that the quality of nadroparin calcium is stable, controllable, efficient and safe.
Owner:BEIJING SAISHENG PHARMA
A kind of preparation method of nadroparin calcium
ActiveCN104072638BReduce the proportion of loss on dryingRaise the ratioSODIUM CATIONUltrafiltration
The invention relates to the field of medicine synthesis and discloses a preparation method of nadroparin calcium. The method of the invention firstly uses the sodium nitrite method to prepare the low-molecular-weight heparin sodium degradation liquid, and then obtains the reducing liquid through reduction. The reducing solution is filtered, the obtained filtrate is stirred evenly with calcium chloride solution and ultrafiltered, the ultrafiltrate is added with ethanol to precipitate, the precipitate is redissolved with water, filtered, the obtained filtrate is sterilized and freeze-dried to obtain the finished product of nadroparin calcium. The preparation process of the present invention provides a brand-new preparation idea, discards the hydrogen peroxide oxidation in the existing method and the calcium and sodium replacement of the cation exchange resin, integrates the ultrafiltration method and calcium chloride stirring to calcium, and cooperates with other optimizations steps, the ratio of anti-Xa / anti-IIa of nadroparin calcium is improved as a whole, the ratio of weight loss on drying of nadroparin calcium and the ratio of fractions with a molecular weight less than 2000 are improved, the process steps are greatly reduced, and the preparation efficiency is improved.
Owner:ZHAOKE PHARMA HEFEI
A kind of method of producing nadroparin calcium by heparin sodium crude product
ActiveCN104558250BSimple manufacturing processReduce multiple oxidationUltrafiltrationEthanol precipitation
The invention discloses a method for producing nadroparin calcium from crude heparin sodium. The method uses crude heparin sodium as raw material, pretreatment by salt solution process, oxidation, ion exchange resin adsorption, washing, elution, and finally ultrafiltration and freeze-drying to obtain heparin sodium fine product, which is then depolymerized , reduction, graded alcohol precipitation, calcium transfer, and freeze-drying to obtain nadroparin calcium. The invention has the advantages of controlling the quality of nadroparin calcium from source and process, short production cycle, low production energy consumption and high product quality, and is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
A method for detecting nadroparin calcium products
ActiveCN103398960BAvoid interferenceThe instrument is simpleColor/spectral properties measurementsNitrite ionAbsorbance
The invention relates to the technical field of ultraviolet-visible spectrophotometric method detection and particularly relates to a method for rapidly detecting a nadroparin calcium product. The method comprises the step of detecting the concentration of nadroparin calcium in a solution through detecting the absorbance of a mixed solution in a range of 200nm-800nm by a spectrophotometric method, wherein the mixed solution contains fluorescein, the nadroparin calcium and a buffering solution; preferably, the mixed solution is formed by mixing an ethanol solution of the fluorescein, a nadroparin calcium solution, a sodium sulfate solution and the buffering solution. According to the method disclosed by the invention, an applied instrument is simple and the operation is simple and convenient; the concentration of the nadroparin calcium solution can be rapidly determined. The detection method provided by the invention is strong in anti-interference capability and strong in selectivity, and can be used for monitoring whether the contents of nitrite ions and calcium ions in the nadroparin calcium solution seriously exceed the standard or not. According to the detection method provided by the invention, a linear range is 3.0*10<-3>mol / L-4.5*10<-2>mol / L and a detection limit is 1.0*10<-3>mol / L; the detection method is completely applicable to the detection of a nadroparin calcium middle product and a nadroparin calcium final product and can be used for effectively monitoring a medicine production process of the nadroparin calcium.
Owner:广东三生制药有限公司
Solution concentration of nadroparin calcium determined by phenanthroline-zinc sulfate ultraviolet spectroscopy
ActiveCN103149170BRapid Quantitative DetectionImprove anti-interference abilityColor/spectral properties measurementsAnti jammingUltraviolet
The invention discloses solution concentration of nadroparin calcium determined by phenanthroline-zinc sulfate ultraviolet spectroscopy. Under the environment of buffered solution, Ultraviolet absorbance degrees of mixed solution which includes Zn2+ions, phenanthroline and the nadroparin calcium are determined, and then the concentration of the nadroparin calcium is determined by quantity. According to the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy, a phenanthroline-zinc sulfate ultraviolet spectroscopy test system is applied, quick quantitative determination of the solution concentration of the nadroparin calcium can be achieved. The linear range of an analytical method of the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy is 1.184%-16.33%, meanwhile, the analytical method of the solution concentration of the nadroparin calcium determined by the phenanthroline-zinc sulfate ultraviolet spectroscopy has good anti-jamming capability and can be applied in the practical production process of nadroparin calcium medicines and capable of carrying out concentration determination to intermediate product solution or finished product solution.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
A kind of method of producing nadroparin calcium from heparin sodium crude product
ActiveCN104163878BEliminates the decolorization stepSimple processSulfate radicalsNadroparin calcium
The invention discloses a method for producing nadroparin calcium from crude heparin sodium. The present invention uses crude heparin sodium as a raw material, after adding water to dissolve, and then precipitates and removes impurities in the crude heparin sodium by centrifugation and secondary salting out, then degrades and reduces with nitrous acid to obtain low-molecular-weight heparin containing free sulfate radicals, and then adds chlorinated Barium removes free sulfate radicals produced by degradation, then uses anion exchange resin, and adjusts appropriate parameters for calcium conversion and final impurity removal, and finally oxidative decolorization, filter sterilization, dehydration and drying to obtain nadroparin calcium raw material. The invention uses crude heparin sodium as a raw material, reduces the production cost, improves the yield of nadroparin calcium by simplifying the process steps, reduces the potency loss of the nadroparin calcium, can ensure the safety of the product, and is convenient for large-scale industrial production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
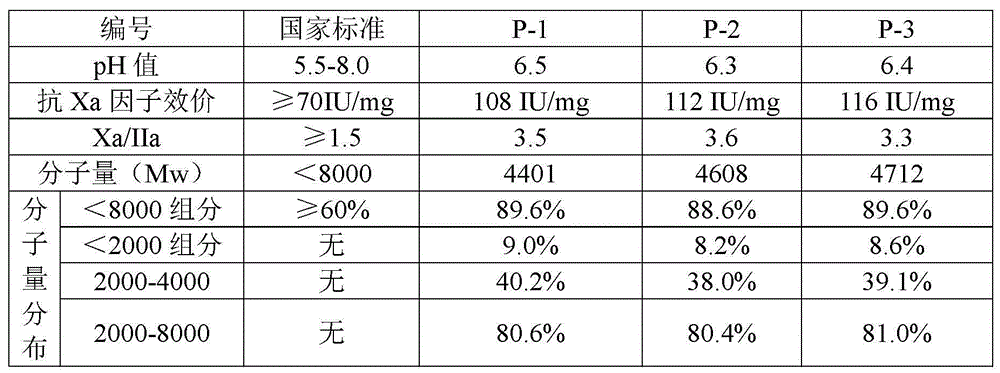
Preparation of high quality nadroparin calcium
The invention relates to the field of biomedicines and in particular relates to a preparation method of high quality nadroparin calcium. The preparation method comprises the steps of degradation, reduction, ethanol precipitation, weak anion exchange chromatographic grading and calcium conversion and filtration, precipitation and dehydration to obtain the high quality nadroparin calcium by taking heparin sodium as a raw material. Compared with the prior art, the molecular weight distribution is controlled more precisely. The anti-Xa factor / anti-II factor potency ratio of a product is higher, and the preparation method is simpler to operate and high in yield and suitable for industrial production.
Owner:WUXI GALAK CHROMATOGRAPHIC TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com