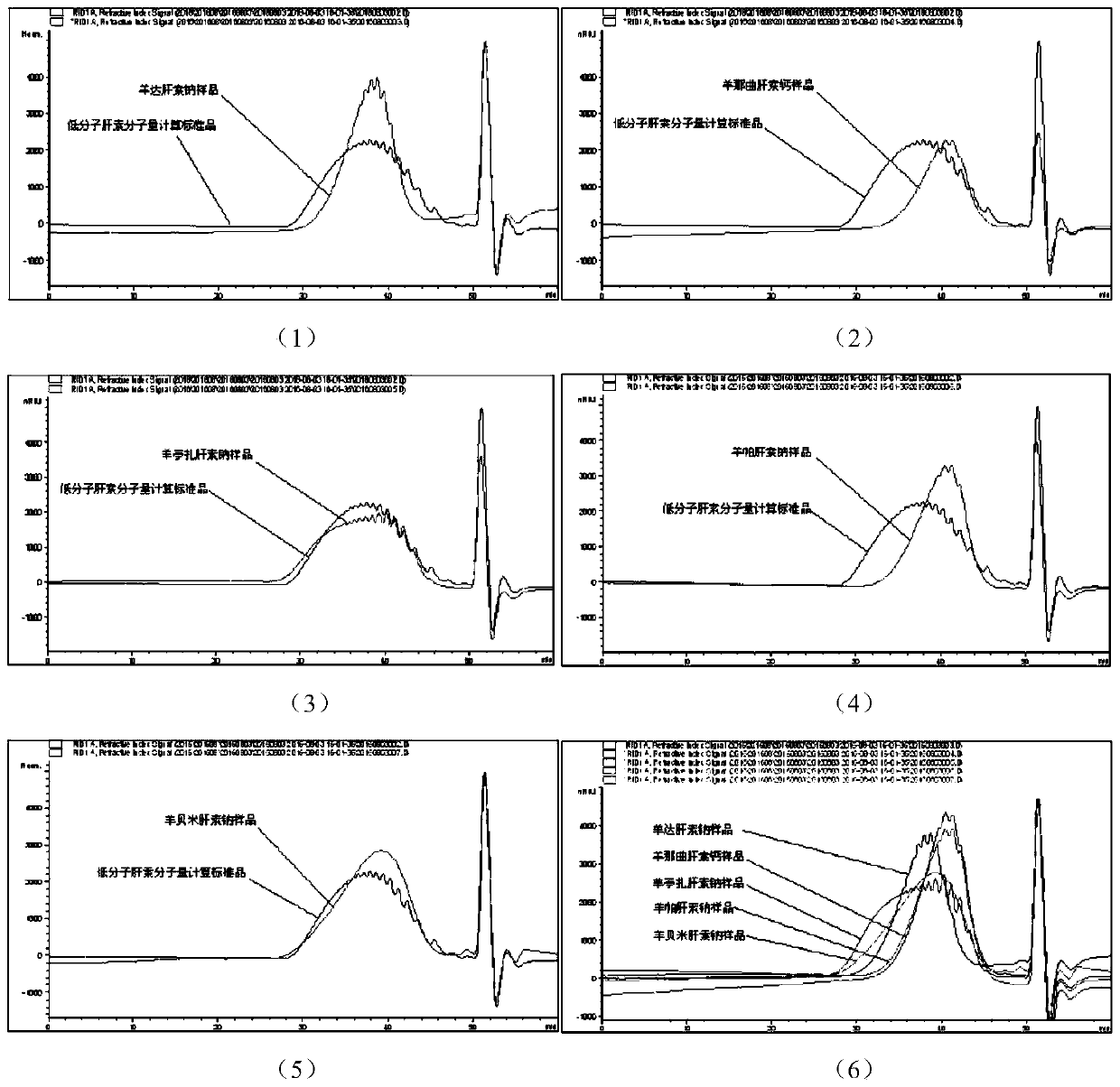
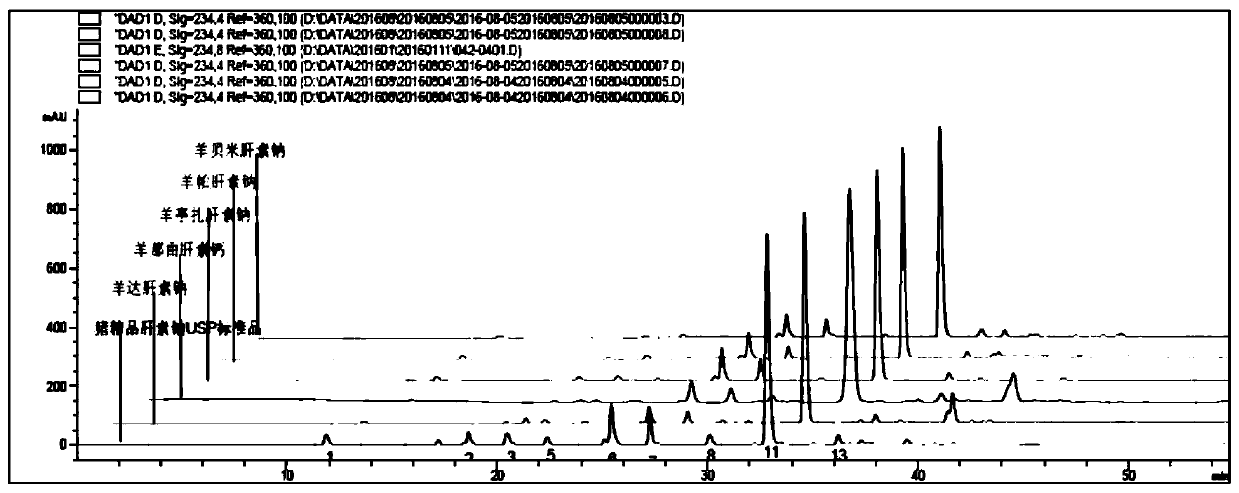
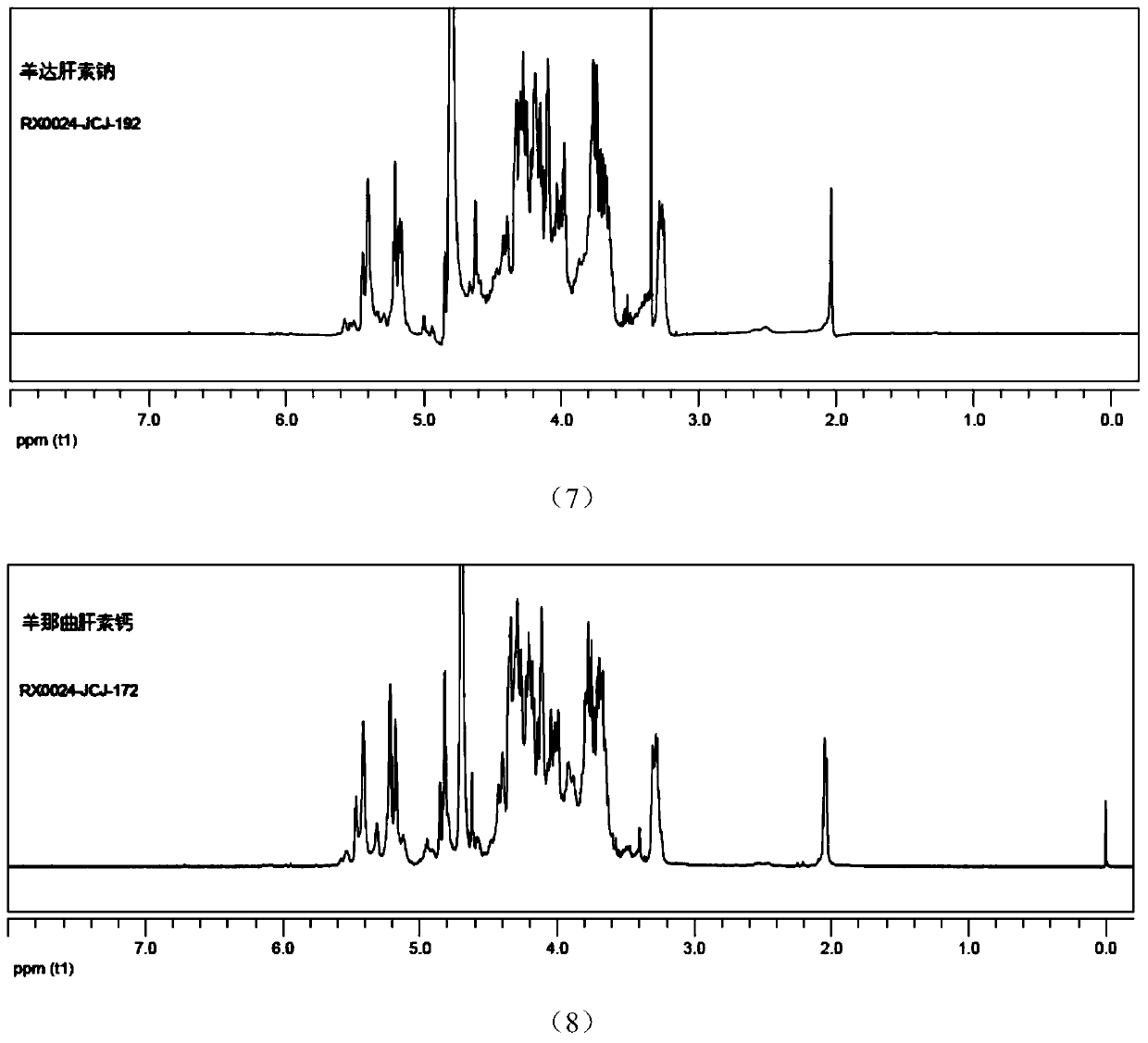
Sheep-derived low-molecular-weight heparin and its preparation method and application
A technology of sheep heparin and sheep paparin sodium, applied in the field of medicine and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Preparation and pretreatment of raw material sheep heparin sodium
[0108] Crude sheep heparin sodium (manufacturer: Shandong Shenlian Biotechnology Co., Ltd., batch number: 20150326), the sample was sent for RT-PCR to detect the DNA content of pigs and sheep. The results showed that the DNA content of pigs was less than 0.1 picogram per microliter, and the DNA content of sheep Greater than 1×10 5 picogram per microliter, and according to the source of the original manufacturer's initial intestinal mucosa, it is determined to be crude sheep heparin sodium, and basically does not contain porcine heparin. In addition, the anticoagulant activity of the sample submitted to the whole sheep plasma method was 67.0 units per mg.
[0109] The purification of crude sheep heparin sodium adopts the well-known process in this industry, that is, it is dissolved in water, followed by salt and alkali hydrolysis, and then hydrolyzed with protease. Sheep heparin, the raw material for p...
Embodiment 2
[0112] Preparation of sheep daparin sodium
[0113] Weigh 50.0 g of the pretreated sheep heparin in Example 1, add 500 ml of purified water, and stir to dissolve the heparin. Control the room temperature in a water bath, and continue to stir and keep warm for more than 30 minutes. Adjust the pH of the heparin solution to 3.1 with hydrochloric acid, add 1.43 g of sodium nitrite, and continue to insulate and stir. Then adjust the pH to neutral, add 4.0 g of sodium borohydride, and continue stirring. Then adjust the pH to neutral with dilute hydrochloric acid, add 51.5 g of sodium chloride, and continue stirring at room temperature for more than 10 minutes. Slowly add 1250 ml of methanol and stir for 15 minutes to precipitate the crude dalteparin obtained in the reaction. The reaction solution was transferred to a centrifuge bottle, and the crude dalteparin precipitate was collected by centrifugation. The precipitate was vacuum-dried and weighed to obtain 43.5 g of crude dalte...
Embodiment 3
[0116] Preparation of Sheep Nadroparin Calcium
[0117] Weigh 50.0 g of the pretreated sheep heparin in Example 1, add 500 ml of purified water, and stir to dissolve the heparin. Control the room temperature in a water bath, and continue to stir and keep warm for more than 30 minutes. Adjust the pH of the heparin solution to 3.0 with hydrochloric acid, add 2.5 g of sodium nitrite, and continue to insulate and stir. Then adjust the pH to neutral, add 4.5 grams of sodium borohydride, and continue stirring. Then adjust the pH to neutral with dilute hydrochloric acid, add 52.0 g of sodium chloride, and continue stirring at room temperature for more than 10 minutes. Slowly add 1250 ml of methanol and stir for 15 minutes to precipitate the crude nadrixarin obtained in the reaction. Transfer the reaction solution to a centrifuge bottle, collect the crude nadrixarin precipitate by centrifugation, and weigh the precipitate after vacuum drying to obtain 39.5 g of the crude nadrixarin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com