Method for preparing nadroparin calcium
A technology of nadroparin calcium and heparin sodium, which is applied in the field of preparation of nadroparin calcium, can solve problems such as unstable product quality, achieve the effects of reducing the risk of pH reduction, good stability, and ensuring controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
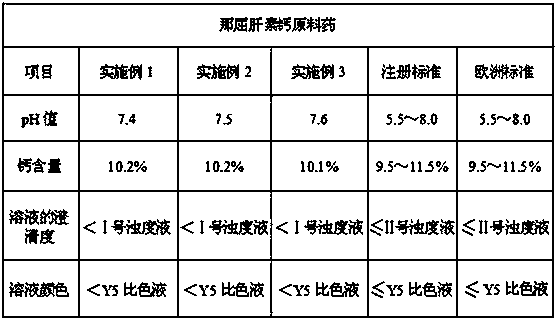
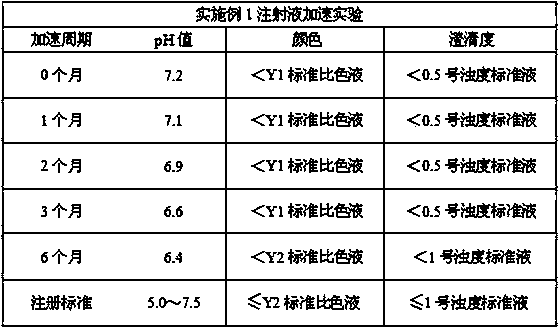
Examples
Embodiment 1
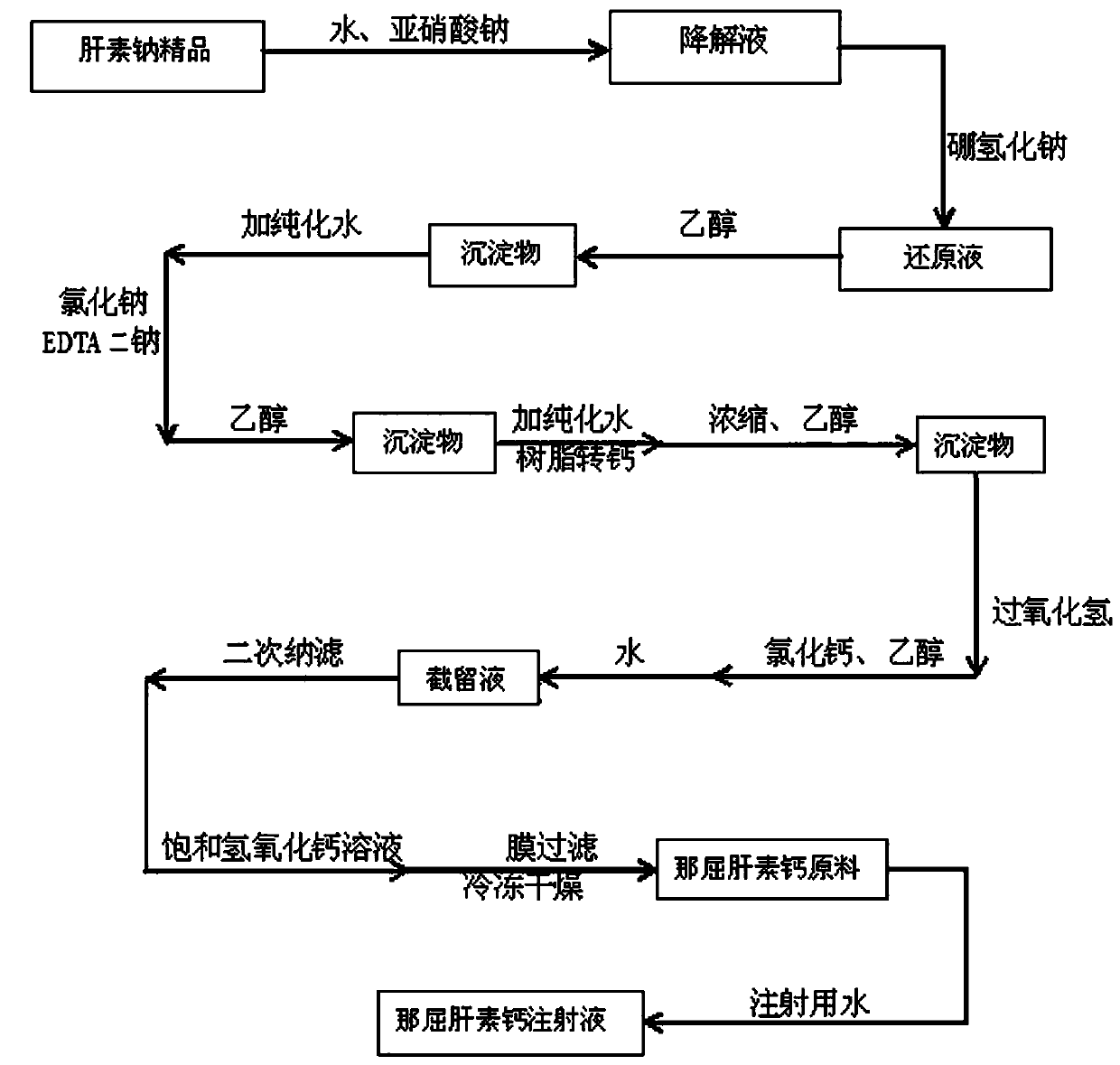
[0028] Embodiment 1: as figure 1 Shown, the preparation method of nadroparin calcium of the present invention comprises following technological process:
[0029] a. Degradation
[0030] Take 3 kg of heparin sodium fine product, add purified water to dissolve it, and then make a constant volume of 30 L. When the temperature of the liquid is adjusted to 20°C, use 20% hydrochloric acid solution to adjust the pH value to 2.0. Weigh 75 g of sodium nitrite and add purified water to dissolve it to a constant volume. 500ml was added to the above-mentioned heparin sodium aqueous solution at a uniform speed for 60 minutes, then kept at 20°C and stirred for 2 hours to obtain a heparin sodium degradation solution;
[0031] b. Restore
[0032] After adjusting the pH value of the above-mentioned heparin sodium degradation solution to 8.5 with 3mol / L sodium hydroxide solution, add 25 g of sodium borohydride at room temperature, stir for 24 hours, adjust the pH value to 4.0 with 20% hydroch...
Embodiment 2
[0045] Embodiment 2: the difference between this embodiment and embodiment 1 is:
[0046] a. Degradation
[0047] Take 3 kg of heparin sodium fine product, add purified water to dissolve it, and then make a constant volume of 20 L. When the temperature of the liquid is adjusted to 19°C, use 20% hydrochloric acid solution to adjust the pH to 3.0. Weigh 90 g of sodium nitrite and add purified water to dissolve it to a constant volume. 500ml was added to the above-mentioned heparin sodium aqueous solution at a uniform speed for 55 minutes, and then kept at a temperature of 19°C, and stirred for 2 hours to obtain a heparin sodium degradation solution;
[0048] b. Restore
[0049] After adjusting the pH value of the heparin sodium degradation solution to 9.0 with 3M sodium hydroxide solution, add 30 g of sodium borohydride at room temperature, stir for 20 hours, adjust the pH value to 4.3 with 20% hydrochloric acid solution, stir for another 30 minutes, and then use 3M sodium hydr...
Embodiment 3
[0062] Embodiment 3: the differences between this embodiment and embodiment 1 are:
[0063] a. Degradation
[0064] Take 3 kg of heparin sodium fine product, add purified water to dissolve it, and then make a constant volume of 15 L. Adjust the temperature of the liquid to 18°C and adjust the pH value to 3.5 with 20% hydrochloric acid solution. Weigh 105 g of sodium nitrite and add purified water to dissolve it to a constant volume. 420ml was added to the above-mentioned heparin sodium aqueous solution at a uniform speed for 50 minutes, then kept at 18°C and stirred for 2 hours to obtain a heparin sodium degradation solution;
[0065] b. Restore
[0066] After adjusting the pH value of the heparin sodium degradation solution to 9.5 with 3M sodium hydroxide solution, add 35 g of sodium borohydride at room temperature, stir for 15 hours, adjust the pH value to 4.5 with 20% hydrochloric acid solution, stir for another 30 minutes, and then use 3M sodium hydroxide Adjust the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com