Production method for nadroparin calcium
A production method and technology of heparin calcium, applied in the field of biomedicine, can solve the problems of cumbersome operation, reliance on freeze dryers, short process steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 45L of purified water and 5kg of heparin sodium into the degradation tank, stir to dissolve completely, adjust the pH of the solution in the tank to 2.7 with 4mol / L hydrochloric acid, weigh 0.135kg of sodium nitrite into the beaker, and add 1350mL of purified water into the beaker , after stirring and dissolving, add it into the degradation tank, maintain the pH of the solution in the tank at 2.7±0.1, and stir and react at 25±2°C for 3.6h. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 9.6, add 0.054kg sodium borohydride to the degradation tank, stir and react for 18h, then adjust the pH of the solution to 3.5 with 4mol / L hydrochloric acid, stir for 20min, and then use 5mol / L Adjust the pH of the system to 7.3 with sodium hydroxide solution, irradiate the degradation solution with a 254nm ultraviolet lamp for 20 minutes, the solution volume is 48L, so pour 48L of ethanol into the degradation tank, stir at 15-20°C for 5-10min, stop stirring and...
Embodiment 2
[0026] Add 42.5L of purified water and 5kg of heparin sodium into the degradation tank, stir to dissolve completely, adjust the pH of the solution in the tank to 2.6 with 4mol / L hydrochloric acid, weigh 0.10kg of sodium nitrite into the beaker, and add 1000mL of purified Water, after stirring and dissolving, was added into the degradation tank, and the pH of the solution in the tank was maintained at 2.7±0.1, and the reaction was stirred at 25±2°C for 3.4 hours. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 9.5, add 0.03kg sodium borohydride to the degradation tank, stir and react for 17h, then adjust the pH of the solution to 3.4 with 4mol / L hydrochloric acid, stir for 20min, and then use 5mol / L hydrogen Sodium oxide solution adjusts the pH of the system to 7.4, irradiates the degradation solution with a 254nm ultraviolet lamp for 20 minutes, and the solution volume is 44.8L, so pour 44.8L of ethanol into the degradation tank, stir at 15-20°C for 5-10m...
Embodiment 3
[0031] Add 50L of purified water and 5kg of heparin sodium into the degradation tank, stir to dissolve it completely, adjust the pH of the solution in the tank to 2.8 with 4mol / L hydrochloric acid, weigh 0.15kg of sodium nitrite into the beaker, and add 1500mL of purified water into the beaker , after stirring and dissolving, add it into the degradation tank, maintain the pH of the solution in the tank at 2.7±0.1, and stir and react at 25±2°C for 3-4h. Use 5mol / L sodium hydroxide solution to adjust the pH of the system to 10.5, add 0.075kg sodium borohydride to the degradation tank, stir and react for 16 hours, then adjust the pH of the solution to 3.6 with 4mol / L hydrochloric acid, stir for 20min, and then use 5mol / L Sodium hydroxide solution adjusts the pH of the system to 7.5, irradiates the degradation solution with a 254nm ultraviolet lamp for 20 minutes, and the solution volume is 53.6L, so pour 53.6L of ethanol into the degradation tank, stir at 15-20°C for 5-10min, stop...
PUM
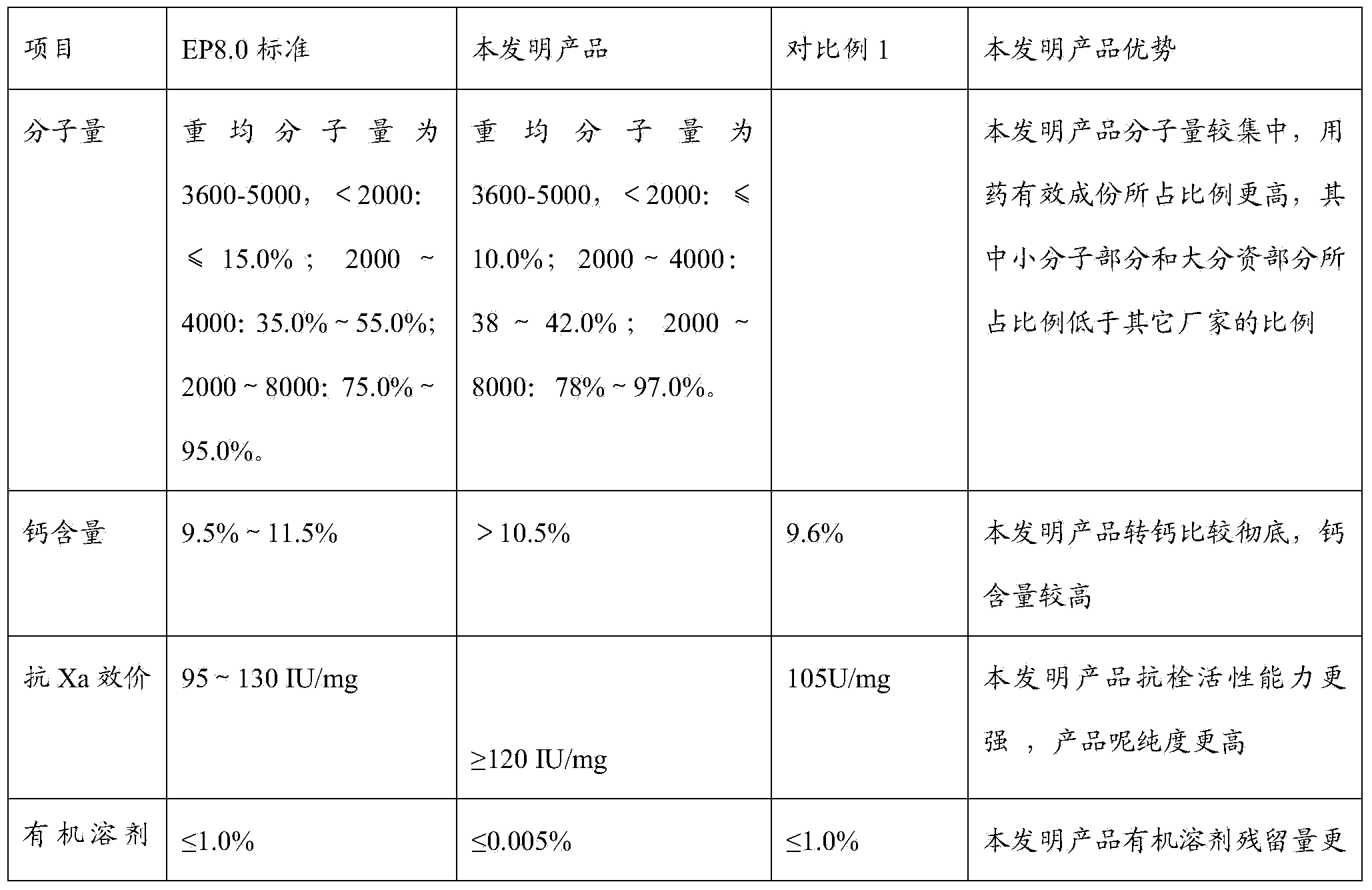
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com