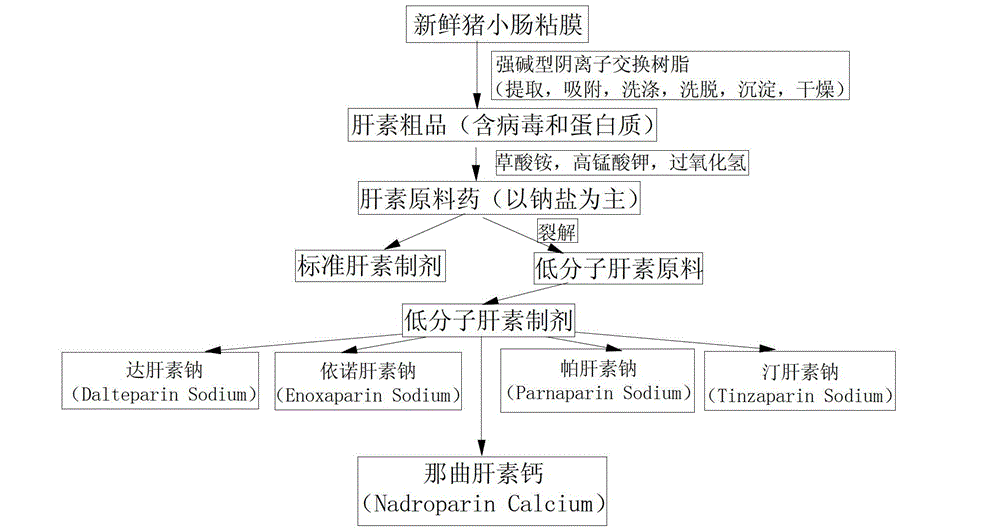
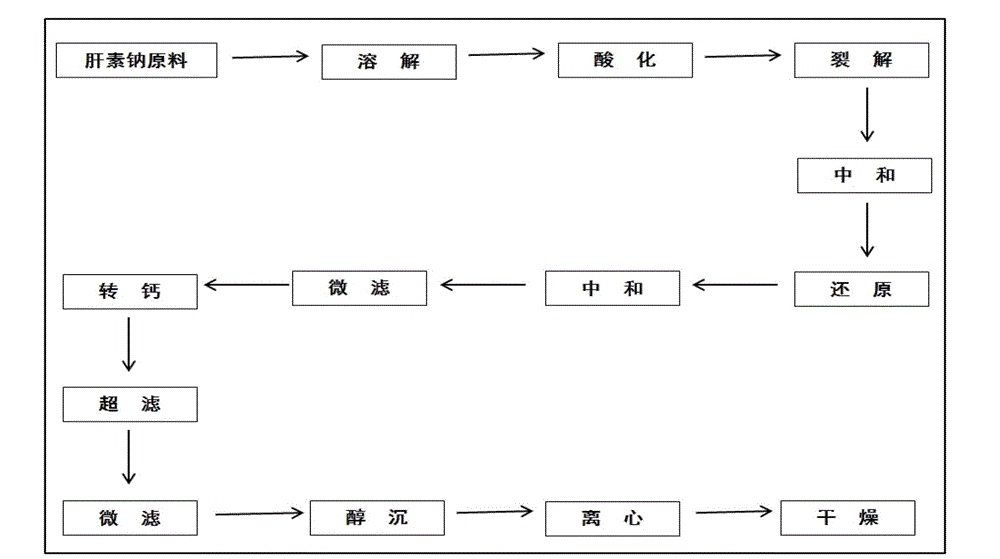
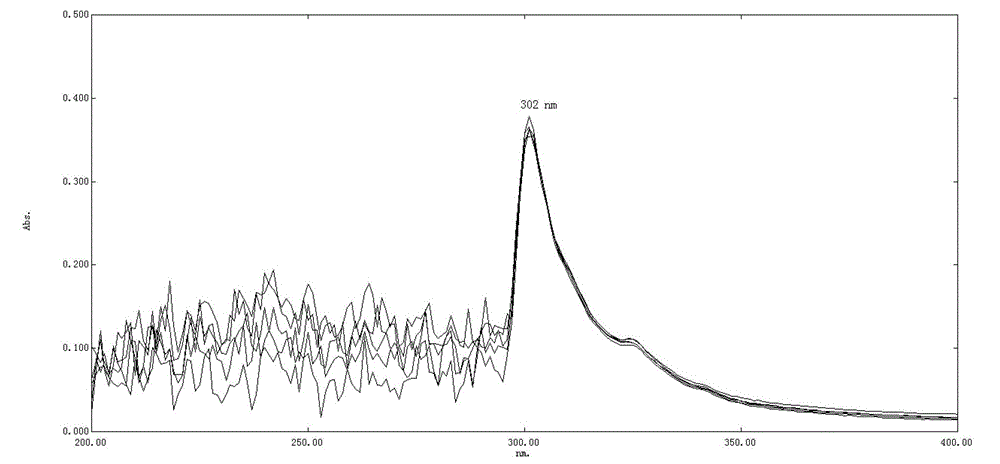
Solution concentration of nadroparin calcium determined by phenanthroline-zinc sulfate ultraviolet spectroscopy
A technology of phenanthroline and ultraviolet spectroscopy, which is applied in the field of rapid quantitative determination of the concentration of nadroparin calcium solution, can solve problems such as inability to apply and large analytical errors, and achieve the effect of good anti-interference ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] The embodiment discloses the determination of the solution concentration of nadroparin calcium by o-phenanthroline-zinc sulfate ultraviolet spectroscopy. First, the applicability of the o-phenanthroline-zinc sulfate system, the anti-interference ability to calcium ions, and the The influence of the dosage of sodium acetate solution, the influence of the dosage of o-phenanthroline and the influence of the dosage of sodium nitrite solution, to illustrate the applicability, variable range and detection principle of the detection system; Concentration standard working curve, Experiment 7 verified the reliability of the method and the standard working curve through experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com